Abstract
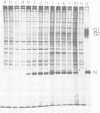
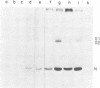
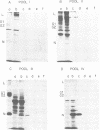
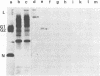
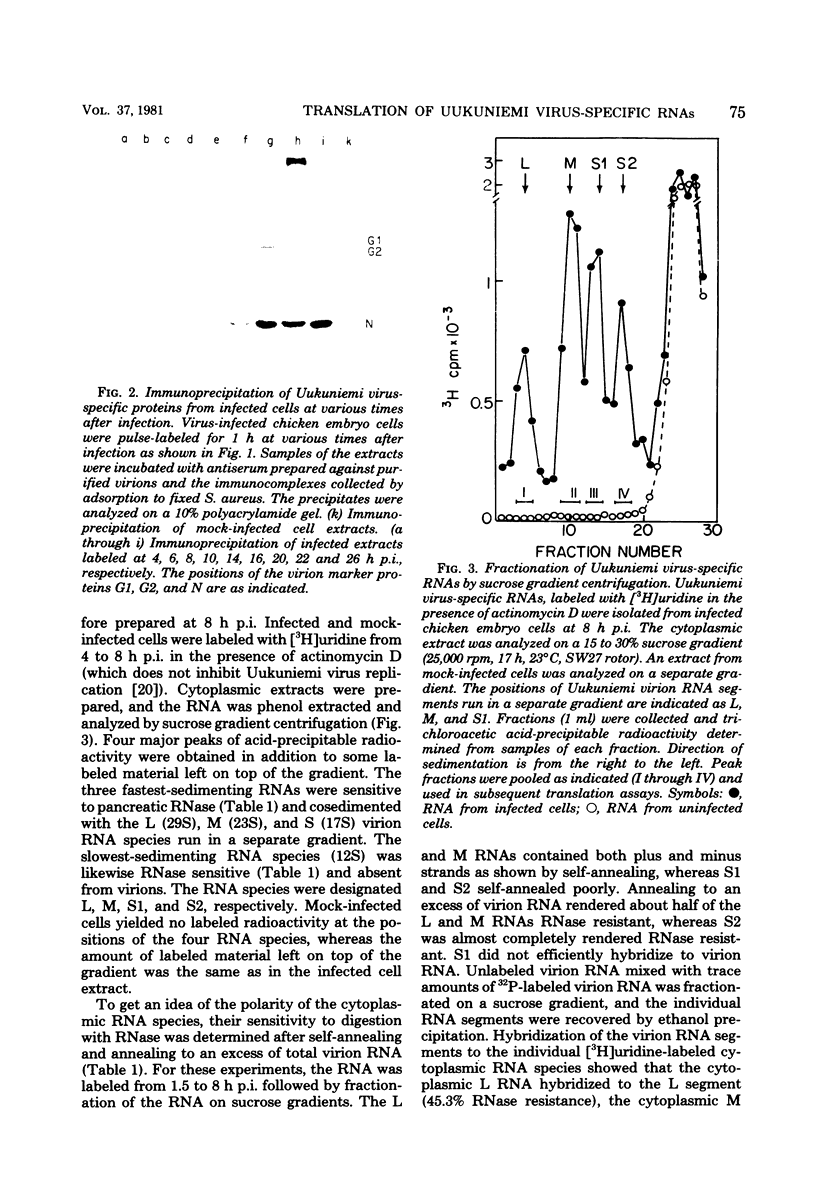
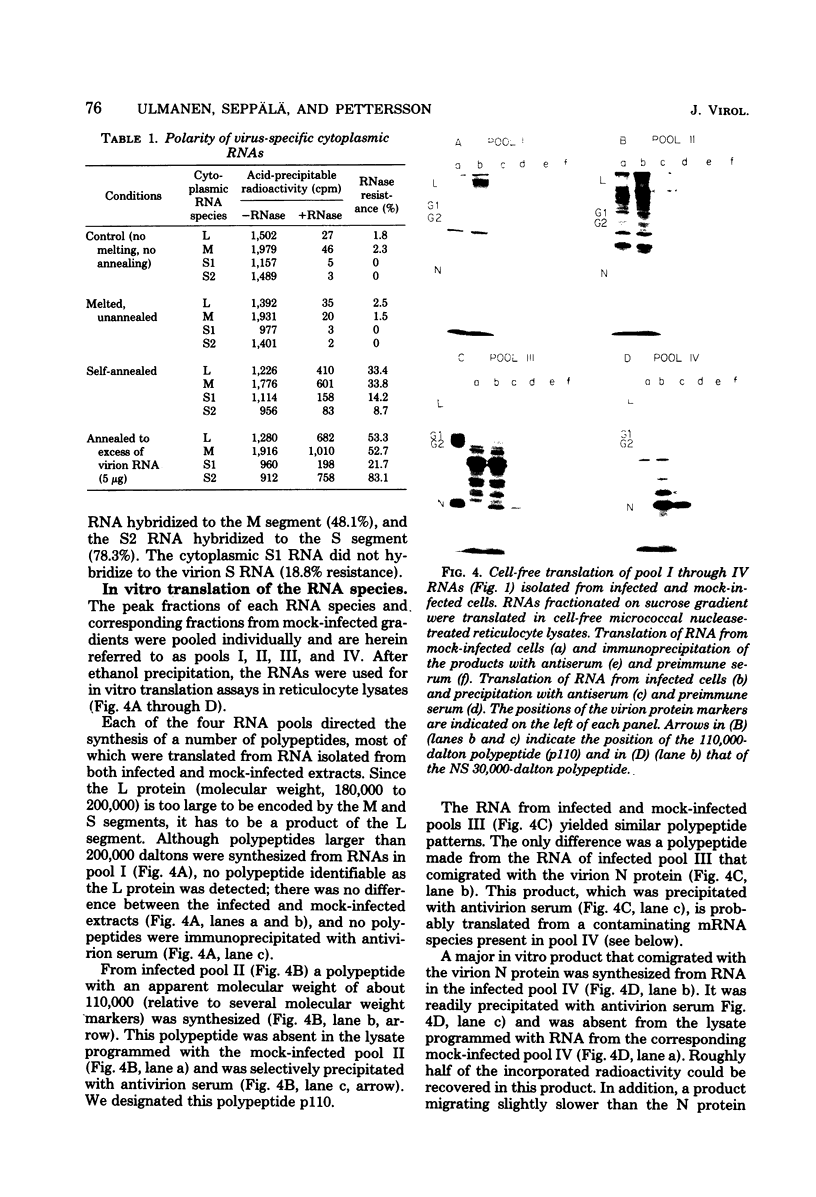
We isolated the virus-specific RNA species from Uukuniemi virus-infected chicken embryo cells and fractionated them by sucrose gradient centrifugation. In addition to three RNA species cosedimenting with the three viral RNA segments L (29S), M (23S), and S (17S), a fourth major RNA species, sedimenting at about 12S (S2), was found early in the infection. Annealing experiments indicated that the cytoplasmic L and M RNA species consisted of both plus and minus strands, with the plus strands in slight excess. Most of the S1 RNA was of negative polarity, whereas S2 was of positive polarity. The S2 RNA specifically annealed to the virion S RNA segment, indicating that it is transcribed from this segment. In vitro translation of the individual RNA species in micrococcal nuclease-treated cell-free reticulocyte extracts showed that an mRNA cosedimenting with the virion M RNA directed the synthesis of a virus-specific 110,000-dalton polypeptide (p110). This polypeptide could be immunoprecipitated with antiserum prepared against purified virions. When translation was carried out in the presence of dog pancreas microsomes, p110 was absent. Instead, an immunoprecipitable polypeptide band, with a molecular weight of about 70,000 and migrating between the virion surface glycoproteins G1 and G2, was observed. It is thus likely that the glycoproteins are synthesized as a precursor (p110), which during translation is cleaved roughly in the middle to yield G1 and G2. The 12S RNA species directed the synthesis of the nucleocapsid protein and a novel polypeptide with an apparent molecular weight of about 30,000. The latter was not precipitated with antivirion serum and was absent from lysates programmed with the corresponding RNA fraction from a mock-infected extract. Since, in addition, it was not found in purified virions and was present in the cytoplasm of infected cells but not in uninfected cells, it probably represents a nonstructural polypeptide.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Hannoun C. Studies on lumbo virus replication. I. RNA-dependent RNA polymerase associated with virions. Virology. 1976 Jan;69(1):258–264. doi: 10.1016/0042-6822(76)90212-9. [DOI] [PubMed] [Google Scholar]
- Bouloy M., Krams-Ozden S., Horodniceanu F., Hannoun C. Three-segment RNA genome of Lumbo virus (Bunyavirus). Intervirology. 1973;2(3):173–180. doi: 10.1159/000149420. [DOI] [PubMed] [Google Scholar]
- Cash P., Vezza A. C., Gentsch J. R., Bishop D. H. Genome complexities of the three mRNA species of snowshoe hare bunyavirus and in vitro translation of S mRNA to viral N polypeptide. J Virol. 1979 Sep;31(3):685–694. doi: 10.1128/jvi.31.3.685-694.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewley J., Gentsch J., Bishop D. H. Three unique viral RNA species of snowshoe hare and La Crosse bunyaviruses. J Virol. 1977 May;22(2):459–468. doi: 10.1128/jvi.22.2.459-468.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder K. T., Bye J. M., Skehel J. J., Waterfield M. D., Smith A. E. In vitro synthesis, glycosylation, and membrane insertion of influenza virus haemagglutinin. Virology. 1979 Jun;95(2):343–350. doi: 10.1016/0042-6822(79)90489-6. [DOI] [PubMed] [Google Scholar]
- Garoff H., Simons K., Dobberstein B. Assembly of the Semliki Forest virus membrane glycoproteins in the membrane of the endoplasmic reticulum in vitro. J Mol Biol. 1978 Oct 5;124(4):587–600. doi: 10.1016/0022-2836(78)90173-0. [DOI] [PubMed] [Google Scholar]
- Gentsch J. R., Bishop D. H. Small viral RNA segment of bunyaviruses codes for viral nucleocapsid protein. J Virol. 1978 Oct;28(1):417–419. doi: 10.1128/jvi.28.1.417-419.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J. R., Bishop D. L. M viral RNA segment of bunyaviruses codes for two glycoproteins, G1 and G2. J Virol. 1979 Jun;30(3):767–770. doi: 10.1128/jvi.30.3.767-770.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J. Studies on the formation of the influenza virus envelope. Virology. 1974 Aug;60(2):398–418. doi: 10.1016/0042-6822(74)90335-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W., Chanock R. M., Lai C. J. Mapping of the two overlapping genes for polypeptides NS1 and NS2 on RNA segment 8 of influenza virus genome. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1857–1861. doi: 10.1073/pnas.77.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Segment 8 of the influenza virus genome is unique in coding for two polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4908–4912. doi: 10.1073/pnas.76.10.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijeski J. F., Bishop D. H., Murphy F. A., Palmer E. L. Structural proteins of La Crosse virus. J Virol. 1976 Sep;19(3):985–997. doi: 10.1128/jvi.19.3.985-997.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijeski J. F., Murphy F. A. Bunyaviridae: recent biochemical developments. J Gen Virol. 1977 Oct;37(1):1–14. doi: 10.1099/0022-1317-37-1-1. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Persson H., Pettersson U., Mathews M. B. Synthesis of a structural adenovirus polypeptide in the absence of viral DNA replication. Virology. 1978 Oct 1;90(1):67–79. doi: 10.1016/0042-6822(78)90334-3. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F. Effect of Uukuniemi virus infection on host cell macromolecule synthesis. Med Biol. 1974 Apr;52(2):90–97. [PubMed] [Google Scholar]
- Pettersson R. F., Hewlett M. J., Baltimore D., Coffin J. M. The genome of Uukuniemi virus consists of three unique RNA segments. Cell. 1977 May;11(1):51–63. doi: 10.1016/0092-8674(77)90316-6. [DOI] [PubMed] [Google Scholar]
- Pettersson R., Käriäinen L. The ribonucleic acids of Uukuniemi virus, a noncubical tick-borne arbovirus. Virology. 1973 Dec;56(2):608–619. doi: 10.1016/0042-6822(73)90062-7. [DOI] [PubMed] [Google Scholar]
- Pettersson R., Käriäinen L., von Bonsdorff C. H., Oker-Blom N. Structural components of Uukuniemi virus, a noncubical tick-borne arbovirus. Virology. 1971 Dec;46(3):721–729. doi: 10.1016/0042-6822(71)90074-2. [DOI] [PubMed] [Google Scholar]
- Ranki M., Pettersson R. F. Uukuniemi virus contains an RNA polymerase. J Virol. 1975 Dec;16(6):1420–1425. doi: 10.1128/jvi.16.6.1420-1425.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., McDowell M., Baltimore D., Lodish H. F. Translation of reovirus mRNA, poliovirus RNA and bacteriophage Qbeta RNA in cell-free extracts of mammalian cells. Methods Enzymol. 1974;30:709–723. doi: 10.1016/0076-6879(74)30068-7. [DOI] [PubMed] [Google Scholar]
- Wirth D. F., Katz F., Small B., Lodish H. F. How a single Sindbis virus mRNA directs the synthesis of one soluble protein and two integral membrane glycoproteins. Cell. 1977 Feb;10(2):253–263. doi: 10.1016/0092-8674(77)90219-7. [DOI] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Pettersson R. Surface structure of Uukuniemi virus. J Virol. 1975 Nov;16(5):1296–1307. doi: 10.1128/jvi.16.5.1296-1307.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]