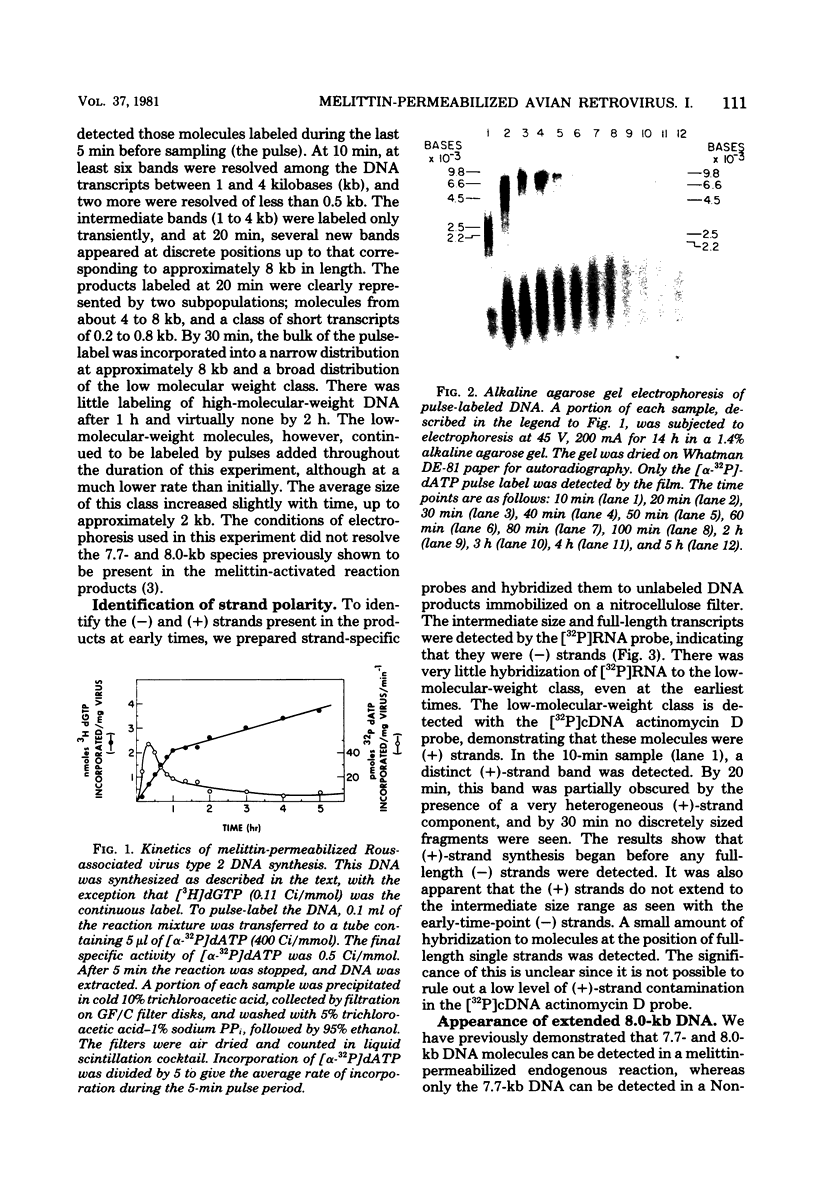
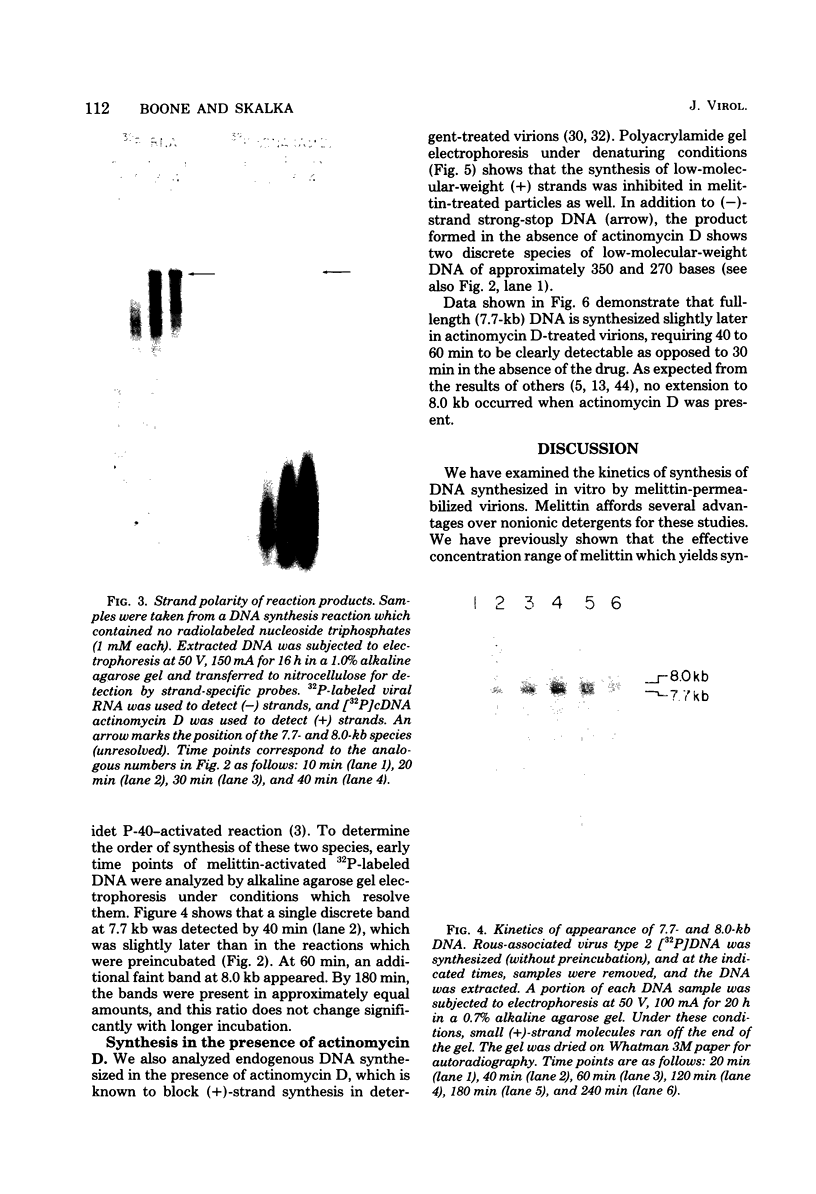
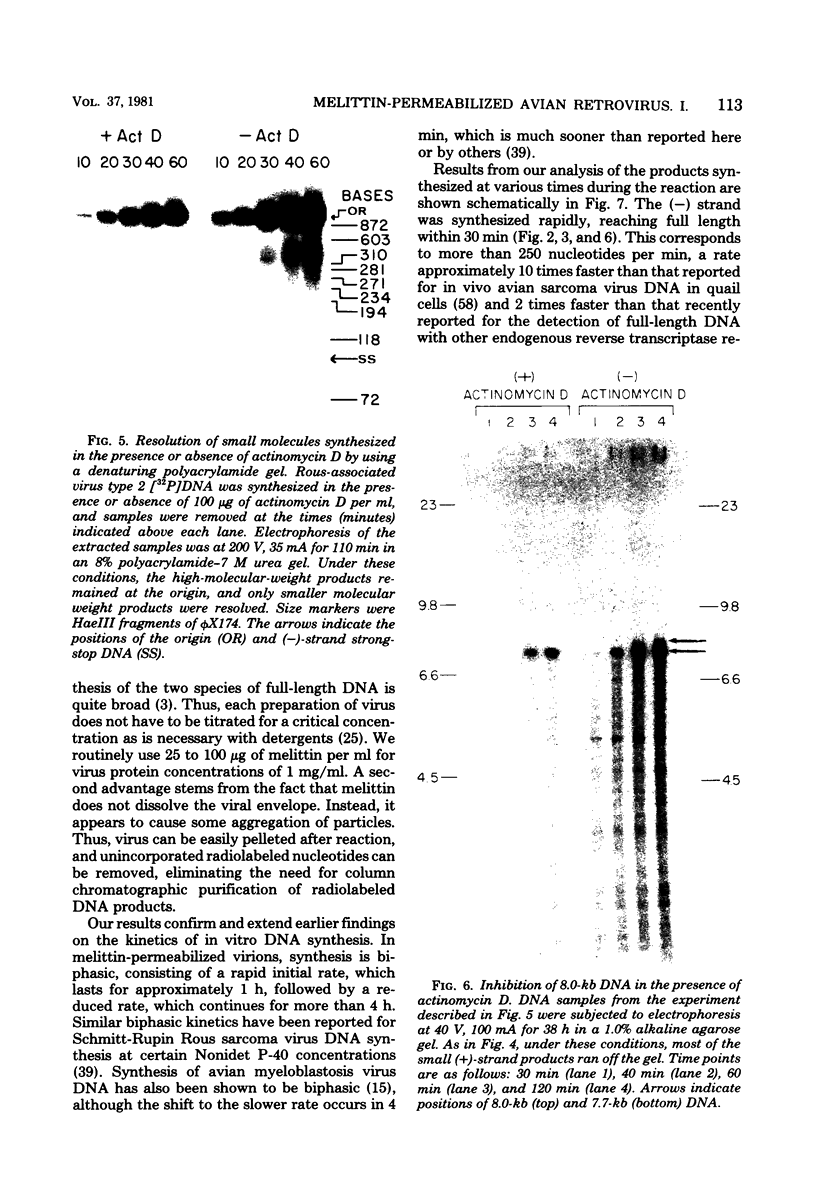
Abstract
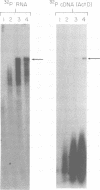
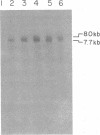
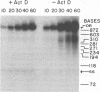
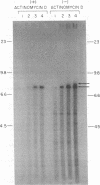
We have examined the kinetics of synthesis of minus [(-)]- and plus [(+)]-strand viral DNA in melittin-permeabilized avian retrovirus particles. The reaction was biphasic. There was a very rapid initial rate, followed, after approximately 1 h, by a lower rate. Many discrete bands of subgenomic-length (-) strands were produced after 10 and 20 min of synthesis; genome-length (7.7-kilobase [kb]) (-) strands were detected within 30 min. Extension to an 8.0-kb (-)-strand species was evident by 60 min. This extension was inhibited by actinomycin D. Synthesis of (+) strands (which is also inhibited by actinomycin D) began early, before any (-) strands were completed, and continued for more than 4 h beyond the time when synthesis of full-length DNA had terminated. Two distinct species of (+)-strand DNA, 0.27 and 0.35 kb, could be observed at the earliest times. Their presence was quickly obscured by subsequent formation of (+)-strand molecules of molecular length between 0.2 and 2.0 kb.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz E. W., Jr, Dina D. Moloney murine sarcoma virions synthesize full-genome-length double-stranded DNA in vitro. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3294–3298. doi: 10.1073/pnas.76.7.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestor T. H., Wang C. S. In vitro synthesis of a 2.1 x 10(6) dalton DNA in the endogenous retrovirus reverse transcriptase reaction. Biochem Biophys Res Commun. 1976 Sep 7;72(1):251–257. doi: 10.1016/0006-291x(76)90987-6. [DOI] [PubMed] [Google Scholar]
- Boone L. R., Skalka A. M. Viral DNA synthesized in vitro by avian retrovirus particles permeabilized with melittin. II. Evidence for a strand displacement mechanism in plus-strand synthesis. J Virol. 1981 Jan;37(1):117–126. doi: 10.1128/jvi.37.1.117-126.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone L. R., Skalka A. Two species of full-length cDNA are synthesized in high yield by melittin-treated avian retrovirus particles. Proc Natl Acad Sci U S A. 1980 Feb;77(2):847–851. doi: 10.1073/pnas.77.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosselman R. A., Verma I. M. Genome organization of retroviruses. V. In vitro-synthesized Moloney murine leukemia viral DNA has long terminal redundancy. J Virol. 1980 Jan;33(1):487–493. doi: 10.1128/jvi.33.1.487-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Duesberg P., Dina D. Cleavage map of linear mouse sarcoma virus DNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):29–33. doi: 10.1073/pnas.74.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayman C. H., Mosharrafa E., Faras A. J. In vitro synthesis of infectious transforming DNA by the avian sarcoma virus reverse transcriptase. J Virol. 1979 Jan;29(1):242–249. doi: 10.1128/jvi.29.1.242-249.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Dierks P., Parsons J. T., Faras A. J. RNase H hydrolysis of the 5' terminus of the avian sarcoma virus genome during reverse transcription. Nature. 1978 Mar 9;272(5649):181–184. doi: 10.1038/272181a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. Evidence for circularization of the avian oncornavirus RNA genome during proviral DNA synthesis from studies of reverse transcription in vitro. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1329–1332. doi: 10.1073/pnas.73.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. In vitro transcription of theavian oncornavirus genome by the RNA-directed DNA polymerase: analysis of DNA transcripts synthesized in reconstructed enzymatic reactions. J Virol. 1977 Apr;22(1):86–96. doi: 10.1128/jvi.22.1.86-96.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Stavnezer E., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence that binds primer for DNA synthesis to the avian sarcoma virus genome. J Virol. 1976 Aug;19(2):548–558. doi: 10.1128/jvi.19.2.548-558.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Bromley P. A., Spahr P. F. Extensive in vitro transcription of rous sarcoma virus RNA by avian myeloblastosis virus DNA polymerase and concurrent activation of the associated RNase H. J Virol. 1977 Sep;23(3):659–668. doi: 10.1128/jvi.23.3.659-668.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina D., Benz E. W., Jr Structure of murine sarcoma virus DNA replicative intermediates synthesized in vitro. J Virol. 1980 Jan;33(1):377–389. doi: 10.1128/jvi.33.1.377-389.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich R., Moelling K. Effect of viral RNase H on the avian sarcoma viral genome during early transcription in vitro. J Virol. 1979 Sep;31(3):630–638. doi: 10.1128/jvi.31.3.630-638.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Parsons J. T., Beard J. W., Beard D., Green M. Mechanism of carcinogenesis by RNA tumor viruses. 3. Formation of RNA, DNA complex and duplex DNA molecules by the DNA polymerase (s) of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1432–1439. doi: 10.1073/pnas.67.3.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni A. M., Smotkin D., Weinberg R. A. Murine leukemia virus: detection of unintegrated double-stranded DNA forms of the provirus. Proc Natl Acad Sci U S A. 1975 Feb;72(2):447–451. doi: 10.1073/pnas.72.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni A. M., Weinberg R. A. Partially single-stranded form of free Moloney viral DNA. Nature. 1975 Jun 19;255(5510):646–648. doi: 10.1038/255646a0. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Goff S., Shields A., Yoshimura F., Mitra S., Baltimore D. In vitro synthesis of a 9 kbp terminally redundant DNA carrying the infectivity of Moloney murine leukemia virus. Cell. 1979 Apr;16(4):863–874. doi: 10.1016/0092-8674(79)90101-6. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Mitra S. W., Goff S., Baltimore D. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979 Sep;18(1):93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Guntaka R. V., Mahy B. W., Bishop J. M., Varmus H. E. Ethidium bromide inhibits appearance of closed circular viral DNA and integration of virus-specific DNA in duck cells infected by avian sarcoma virus. Nature. 1975 Feb 13;253(5492):507–511. doi: 10.1038/253507a0. [DOI] [PubMed] [Google Scholar]
- Guntaka R. V. Structure of avian tumor virus DNA intermediates. Biochem Biophys Res Commun. 1978 May 15;82(1):335–341. doi: 10.1016/0006-291x(78)90614-9. [DOI] [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T. W., Sabran J. L., Mark G. E., Guntaka R. V., Taylor J. M. Analysis of unintegrated avian RNA tumor virus double-stranded DNA intermediates. J Virol. 1978 Dec;28(3):810–818. doi: 10.1128/jvi.28.3.810-818.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Duesberg P. H., Knight C. A. In vitro synthesis of full-length DNA transcripts of Rous sarcoma virus RNA by viral DNA polymerase. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4895–4899. doi: 10.1073/pnas.72.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Hu S., Knight C. A., Davidson N. Heteroduplex analysis of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1977 Feb;74(2):477–481. doi: 10.1073/pnas.74.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Myers J. C. Anticomplementary nature of smaller DNA produced during synthesis of extensive DNA copies of poliovirus RNA. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3408–3412. doi: 10.1073/pnas.73.10.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Hu S. S. In vitro synthesis and characterisation of full- and half-genome length complementary DNA from avian oncoviruses. Nature. 1978 Feb 2;271(5644):481–483. doi: 10.1038/271481a0. [DOI] [PubMed] [Google Scholar]
- Lovinger G. G., Klein R., Ling H. P., Gilden R. V., Hatanaka M. Kinetics of murine type C virus-specific DNA synthesis newly infected cells. J Virol. 1975 Oct;16(4):824–831. doi: 10.1128/jvi.16.4.824-831.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly K. F., Smoler D. F., Bromfeld E., Baltimore D. Forms of deoxyribonucleic acid produced by virions of the ribonucleic acid tumor viruses. J Virol. 1971 Jan;7(1):106–111. doi: 10.1128/jvi.7.1.106-111.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements W., Hanafusa H., Tilghman S., Skalka A. Structural studies on oncornavirus-related sequences in chicken genomic DNA: two-step analyses of EcoRI and Bgl I restriction digests and tentative mapping of a ubiquitous endogenous provirus digests and tentative mapping of a ubiquitous endogenous provirus. Proc Natl Acad Sci U S A. 1979 May;76(5):2165–2169. doi: 10.1073/pnas.76.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- McDonnell J. P., Garapin A. C., Levinson W. E., Quintrell N., Fanshier L., Bishop J. M. DNA polymerases of Rous sarcoma virus: delineation of two reactions with actinomycin. Nature. 1970 Oct 31;228(5270):433–435. doi: 10.1038/228433a0. [DOI] [PubMed] [Google Scholar]
- Mitra S. W., Goff S., Gilboa E., Baltimore D. Synthesis of a 600-nucleotide-long plus-strand DNA by virions of Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4355–4359. doi: 10.1073/pnas.76.9.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Dobkin C., Spiegelman S. RNA primer used in synthesis of anticomplementary DNA by reverse transcriptase of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1316–1320. doi: 10.1073/pnas.77.3.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Spiegelman S., Kacian D. L. Synthesis of full-length DNA copies of avian myeloblastosis virus RNA in high yields. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2840–2843. doi: 10.1073/pnas.74.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak U., Friedrich R., Moelling K. Elongation of DNA complementary to the 5' end of the avian sarcoma virus genome by the virion-associated RNA-dependent DNA polymerase. J Virol. 1979 May;30(2):438–452. doi: 10.1128/jvi.30.2.438-452.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Harada F., Dahlberg J. E., Panet A., Haseltine W. A., Baltimore D. Low-molecular-weight RNAs of Moloney murine leukemia virus: identification of the primer for RNA-directed DNA synthesis. J Virol. 1977 Mar;21(3):1031–1041. doi: 10.1128/jvi.21.3.1031-1041.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintrell N., Fanshier L., Evans B., Levinson W., Bishop J. M. Deoxyribonucleic acid polymerase(s) of Rous sarcoma virus: effects of virion-associated endonuclease on the enzymatic product. J Virol. 1971 Jul;8(1):17–27. doi: 10.1128/jvi.8.1.17-27.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., Coggins L. Synthesis of long complementary DNA in the endogenous reaction by equine infectious anemia virus. J Virol. 1979 Mar;29(3):907–914. doi: 10.1128/jvi.29.3.907-914.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Shank P. R., Varmus H. E. Mouse mammary tumor virus DNA in infected rat cells: characterization of unintegrated forms. Cell. 1977 Jan;10(1):19–26. doi: 10.1016/0092-8674(77)90135-0. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Increased length of DNA made by virions of murine leukemia virus at limiting magnesium ion concentration. J Virol. 1977 Jan;21(1):168–178. doi: 10.1128/jvi.21.1.168-178.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Synthesis of long, representative DNA copies of the murine RNA tumor virus genome. J Virol. 1975 Jan;17(1):168–174. doi: 10.1128/jvi.17.1.168-174.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Smotkin D., Baltimore D., Weinberg R. A. In vitro synthesis of infectious DNA of murine leukaemia virus. Nature. 1977 Sep 8;269(5624):122–126. doi: 10.1038/269122a0. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Zamecnik P. C., Weith H. L. Rous sarcoma virus genome is terminally redundant: the 3' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):994–998. doi: 10.1073/pnas.74.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Varmus H. E. Virus-specific DNA in the cytoplasm of avian sarcoma virus-infected cells is a precursor to covalently closed circular viral DNA in the nucleus. J Virol. 1978 Jan;25(1):104–104. doi: 10.1128/jvi.25.1.104-104.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Czernilofsky A. P., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence at the 5' terminus of the avian sarcoma virus genome. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1473–1477. doi: 10.1073/pnas.74.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staskus K. A., Collett M. S., Faras A. J. Initiation of DNA synthesis by the avian oncornavirus RNA-directed DNA polymerase: structural and functional localization of the major species of primer RNA on the oncornavirus genome. Virology. 1976 May;71(1):162–168. doi: 10.1016/0042-6822(76)90102-1. [DOI] [PubMed] [Google Scholar]
- Stoll E., Billeter M. A., Palmenberg A., Weissmann C. Avian myeloblastosis virus RNA is terminally redundant: implications for the mechanism of retrovirus replication. Cell. 1977 Sep;12(1):57–72. doi: 10.1016/0092-8674(77)90185-4. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R. Site on the RNA of an avian sarcoma virus at which primer is bound. J Virol. 1975 Sep;16(3):553–558. doi: 10.1128/jvi.16.3.553-558.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglini E. C., Dube D. K., Surrey S., Loeb L. A. Template recognition and chain elongation in DNA synthesis in vitro. J Mol Biol. 1976 Sep 25;106(3):605–621. doi: 10.1016/0022-2836(76)90254-0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Guntaka R. V., Fan W. J., Heasley S., Bishop J. M. Synthesis of viral DNA in the cytoplasm of duck embryo fibroblasts and in enucleated cells after infection by avian sarcoma virus. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3874–3878. doi: 10.1073/pnas.71.10.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Heasley S., Kung H. J., Oppermann H., Smith V. C., Bishop J. M., Shank P. R. Kinetics of synthesis, structure and purification of avian sarcoma virus-specific DNA made in the cytoplasm of acutely infected cells. J Mol Biol. 1978 Mar 25;120(1):55–82. doi: 10.1016/0022-2836(78)90295-4. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Heasley S., Linn J., Wheeler K. Use of alkaline sucrose gradients in a zonal rotor to detect integrated and unintegrated avian sarcoma virus-specific DNA in cells. J Virol. 1976 May;18(2):574–585. doi: 10.1128/jvi.18.2.574-585.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Shank P. R. Unintegrated viral DNA is synthesized in the cytoplasm of avian sarcoma virus-transformed duck cells by viral DNA polymerase. J Virol. 1976 May;18(2):567–573. doi: 10.1128/jvi.18.2.567-573.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M. Genome organization of RNA tumor viruses. I. In vitro synthesis of full-genome-length single-stranded and double-stranded viral DNA transcripts. J Virol. 1978 Jun;26(3):615–629. doi: 10.1128/jvi.26.3.615-629.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F. K., Weinberg R. A. Restriction endonuclease cleavage of linear and closed circular murine leukemia viral DNAs: discovery of a smaller circular form. Cell. 1979 Feb;16(2):323–332. doi: 10.1016/0092-8674(79)90009-6. [DOI] [PubMed] [Google Scholar]