Abstract
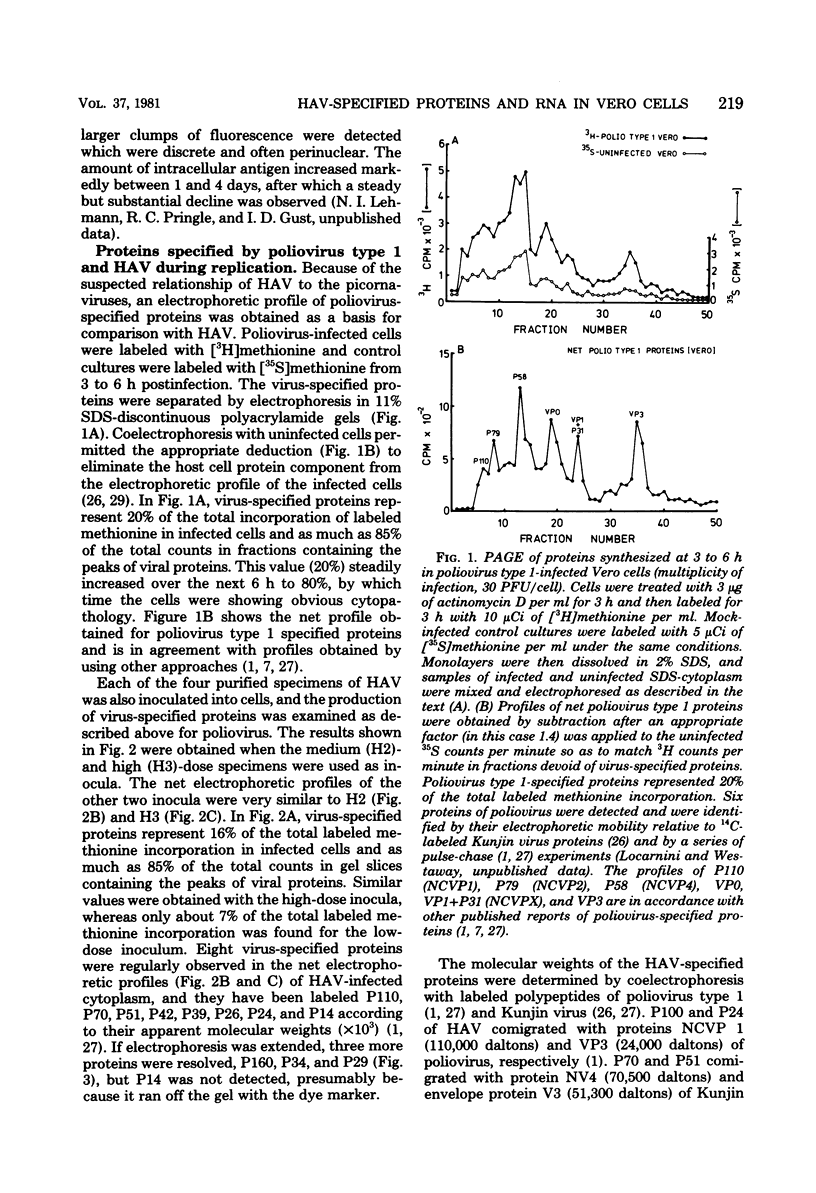
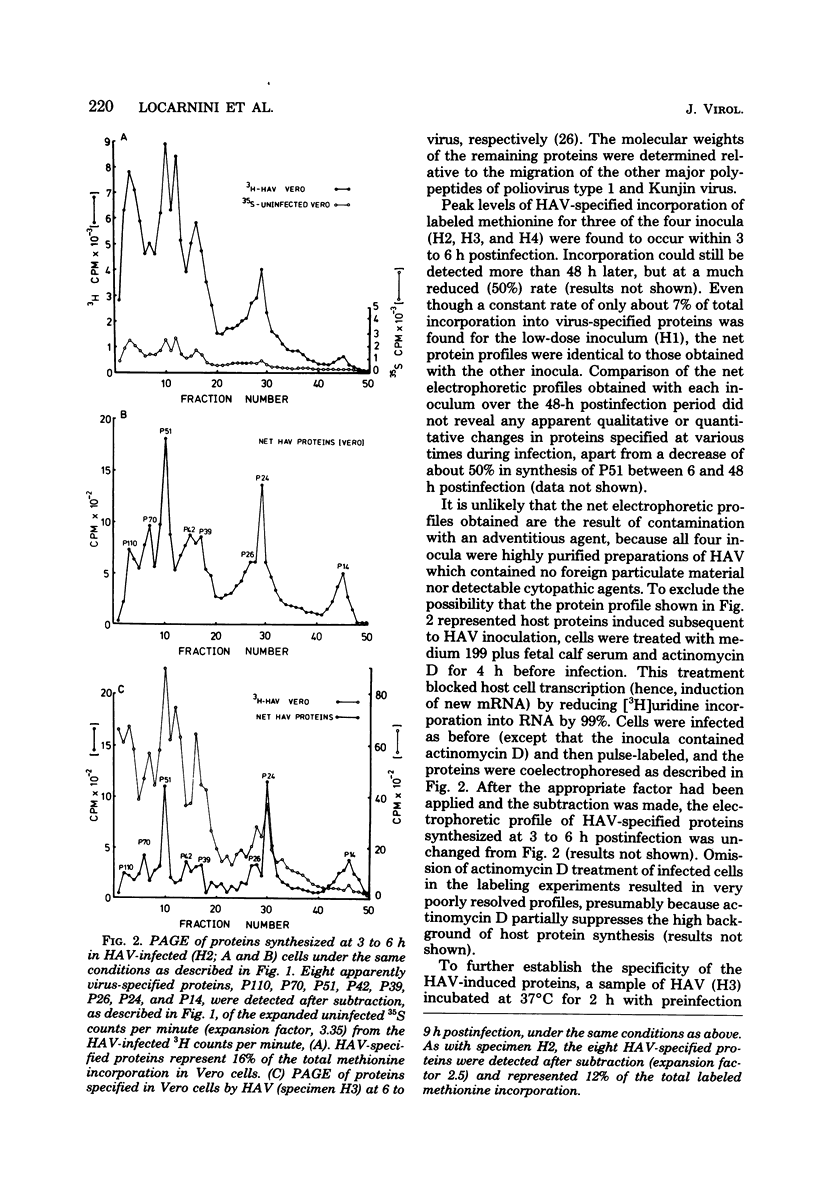
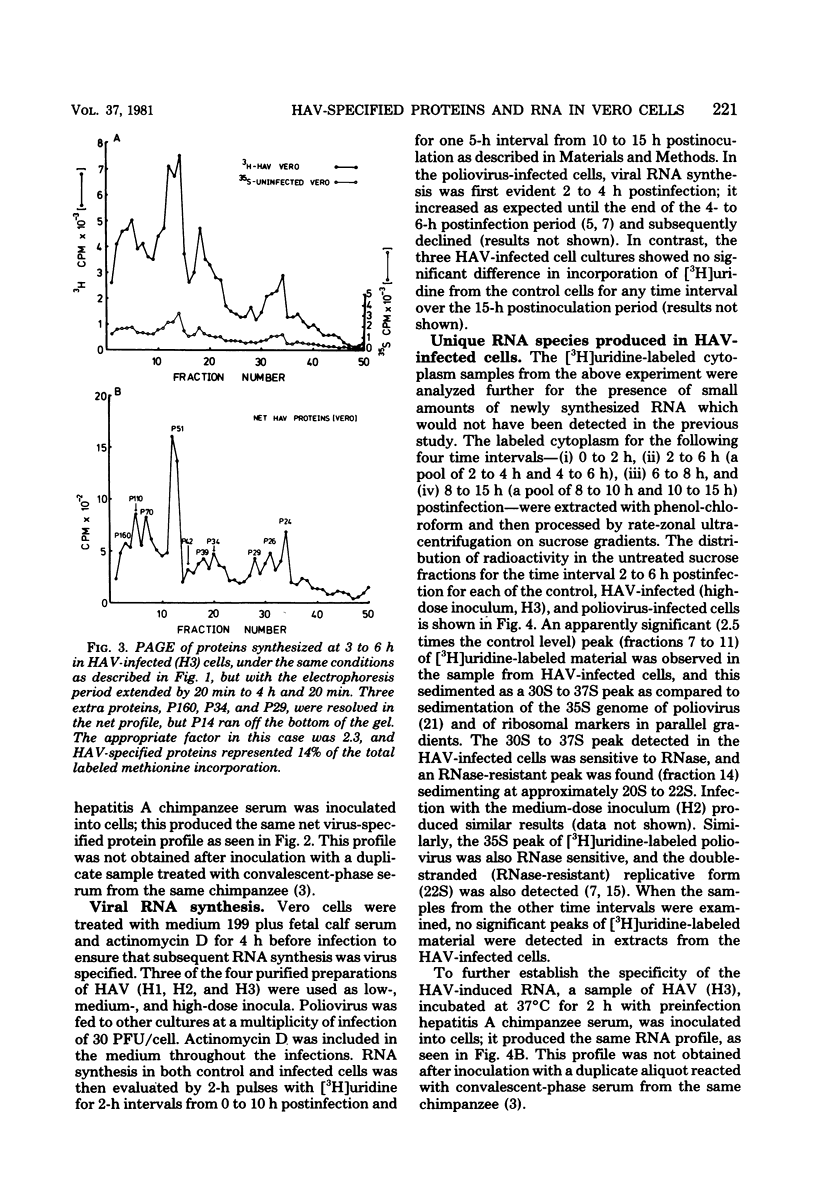
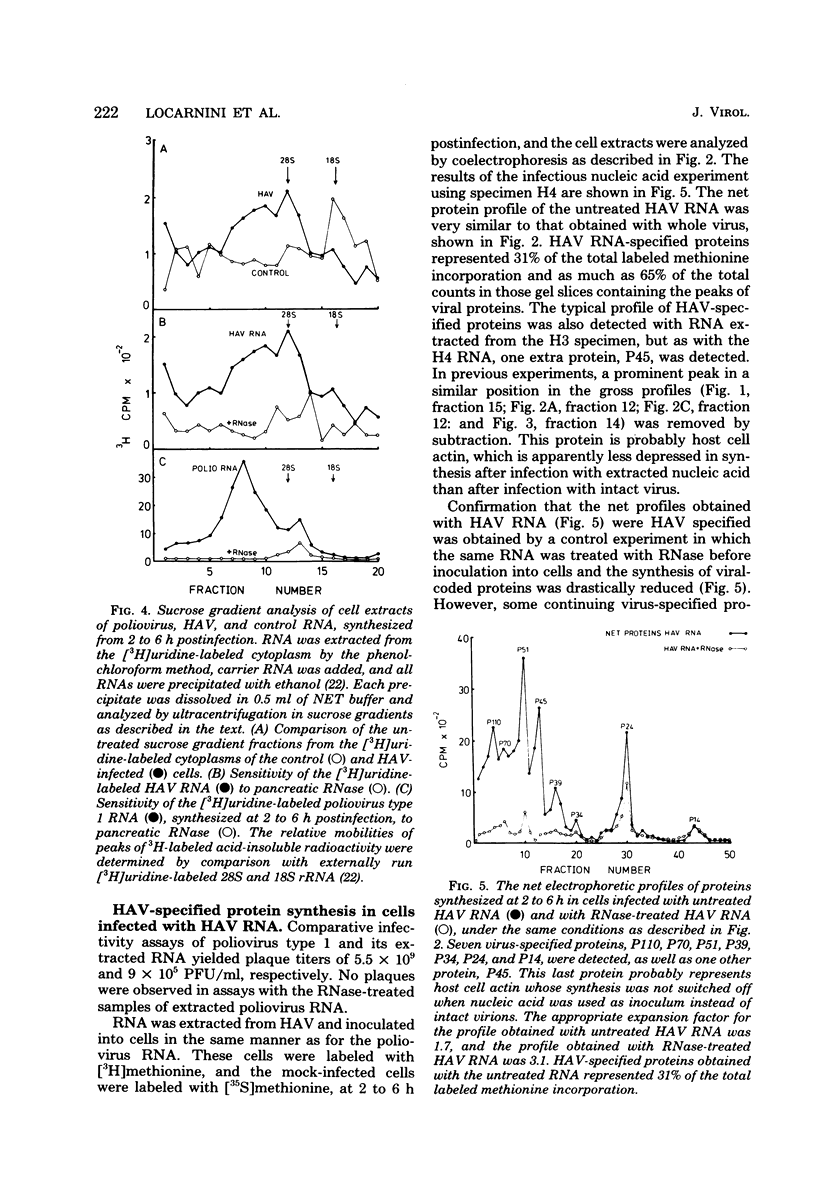
When hepatitis A virus was inoculated into Vero cells, virus-specified protein and RNA synthesis was detected. Production of viral protein was detected by electrophoretic analysis in polyacrylamide gels by using a double-label coelectrophoresis and subtraction method which eliminated the contribution of host protein components from the profiles of virus-infected cytoplasm. Eleven virus-specified proteins were detected in the net electrophoretic profiles of hepatitis A virus-infected cells. The molecular weights of these proteins were very similar to those detected in cells infected with poliovirus type 1. Virus-specified protein synthesis could be detected at 3 to 6 h and continued for at least 48 h postinfection, but no significant effect on host-cell macromolecular synthesis was observed. Limited viral RNA replication occurred between 2 and 6 h postinfection. The genomic RNA of hepatitis A virus was extracted and shown to be capable of infecting cells and inducing the same set of proteins as intact virus, indicating that the RNA genome is positive stranded. Progeny virus was never detected in the supernatant fluids of infected cell cultures, and the cells showed no observable cytopathology, even though hepatitis A virus-specific proteins and antigens were being produced. The nature of the defect in the replicative cycle of hepatitis A virus in this system remains unknown.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Cooper P. D. Poliovirus polypeptides examined in more detail. J Gen Virol. 1975 Nov;29(2):199–213. doi: 10.1099/0022-1317-29-2-199. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K. 5'-terminal cap structure in eucaryotic messenger ribonucleic acids. Microbiol Rev. 1980 Jun;44(2):175–205. doi: 10.1128/mr.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulepis A. G., Locarnini S. A., Ferris A. A., Lehmann N. I., Gust I. D. The polypeptides of hepatitis A virus. Intervirology. 1978;10(1):24–31. doi: 10.1159/000148964. [DOI] [PubMed] [Google Scholar]
- Dienstag J. L., Feinstone S. M., Purcell R. H., Hoofnagle J. H., Barker L. F., London W. T., Popper H., Peterson J. M., Kapikian A. Z. Experimental infection of chimpanzees with hepatitis A virus. J Infect Dis. 1975 Nov;132(5):532–545. doi: 10.1093/infdis/132.5.532. [DOI] [PubMed] [Google Scholar]
- Feinstone S. M., Kapikian A. Z., Purceli R. H. Hepatitis A: detection by immune electron microscopy of a viruslike antigen associated with acute illness. Science. 1973 Dec 7;182(4116):1026–1028. doi: 10.1126/science.182.4116.1026. [DOI] [PubMed] [Google Scholar]
- Helentjaris T., Ehrenfeld E. Inhibition of host cell protein synthesis by UV-inactivated poliovirus. J Virol. 1977 Jan;21(1):259–267. doi: 10.1128/jvi.21.1.259-267.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Locarnini S. A., Coulepis A. G., Ferris A. A., Lehmann N. I., Gust I. D. Purification of hepatitis A virus from human feces. Intervirology. 1978;10(5):300–308. doi: 10.1159/000148992. [DOI] [PubMed] [Google Scholar]
- Locarnini S. A., Coulepis A. G., Stratton A. M., Kaldor J., Gust I. D. Solid-phase enzyme-linked immunosorbent assay for detection of hepatitis A-specific immunoglobulin M. J Clin Microbiol. 1979 Apr;9(4):459–465. doi: 10.1128/jcm.9.4.459-465.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locarnini S. A., Ferris A. A., Stott A. C., Gust I. D. The relationship between a 27-nm virus-like particle and hepatitis A as demonstrated by immune electron microscopy. Intervirology. 1974;4(2):110–118. doi: 10.1159/000149849. [DOI] [PubMed] [Google Scholar]
- Locarnini S. A., Garland S. M., Lehmann N. I., Pringle R. C., Gust I. D. Solid-phase enzyme-linked immunosorbent assay for detection of hepatitis A virus. J Clin Microbiol. 1978 Sep;8(3):277–282. doi: 10.1128/jcm.8.3.277-282.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Koch G. Kinetics of poliovirus replication in HeLa cells infected by isolated RNA. Biochem Biophys Res Commun. 1973 May 15;52(2):635–640. doi: 10.1016/0006-291x(73)90760-2. [DOI] [PubMed] [Google Scholar]
- Pagano J. S. Biologic activity of isolated viral nucleic acids. Prog Med Virol. 1970;12:1–48. [PubMed] [Google Scholar]
- Pagano J. S., Vaheri A. Enhancement of infectivity of poliovirus RNA with diethylaminoethyl-dextran (DEAE-D). Arch Gesamte Virusforsch. 1965;17(3):456–464. doi: 10.1007/BF01241201. [DOI] [PubMed] [Google Scholar]
- Provost P. J., Hilleman M. R. Propagation of human hepatitis A virus in cell culture in vitro. Proc Soc Exp Biol Med. 1979 Feb;160(2):213–221. doi: 10.3181/00379727-160-40422. [DOI] [PubMed] [Google Scholar]
- Provost P. J., Wolanski B. S., Miller W. J., Ittensohn O. L., McAleer W. J., Hilleman M. R. Physical, chemical and morphologic dimensions of human hepatitis A virus strain CR326 (38578). Proc Soc Exp Biol Med. 1975 Feb;148(2):532–539. doi: 10.3181/00379727-148-38578. [DOI] [PubMed] [Google Scholar]
- Rekosh D. Gene order of the poliovirus capsid proteins. J Virol. 1972 Mar;9(3):479–487. doi: 10.1128/jvi.9.3.479-487.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangar D. V. The replication of picornaviruses. J Gen Virol. 1979 Oct;45(1):1–13. doi: 10.1099/0022-1317-45-1-1. [DOI] [PubMed] [Google Scholar]
- Siegl G., Frösner G. G. Characterization and classification of virus particles associated with hepatitis A. II. Type and configuration of nucleic acid. J Virol. 1978 Apr;26(1):48–53. doi: 10.1128/jvi.26.1.48-53.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. A., Gibbs A. J., Cooper P. D. A re-examination of the molecular weight of poliovirus RNA. Biochem Biophys Res Commun. 1970 Jan 23;38(2):298–304. doi: 10.1016/0006-291x(70)90712-6. [DOI] [PubMed] [Google Scholar]
- Tannock G. A., Hierholzer The RNA of human coronavirus OC-43. Virology. 1977 May 15;78(2):500–510. doi: 10.1016/0042-6822(77)90126-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Pagano J. S. Infectious poliovirus RNA: a sensitive method of assay. Virology. 1965 Nov;27(3):434–436. doi: 10.1016/0042-6822(65)90126-1. [DOI] [PubMed] [Google Scholar]
- Wall R., Taylor M. W. Mengovirus RNA synthesis in productive and restrictive cell lines. Virology. 1970 Sep;42(1):78–86. doi: 10.1016/0042-6822(70)90240-0. [DOI] [PubMed] [Google Scholar]
- Westaway E. G. Proteins specified by group B togaviruses in mammalian cells during productive infections. Virology. 1973 Feb;51(2):454–465. doi: 10.1016/0042-6822(73)90444-3. [DOI] [PubMed] [Google Scholar]
- Westaway E. G., Reedman B. M. Proteins of the group B arbovirus Kunjin. J Virol. 1969 Nov;4(5):688–693. doi: 10.1128/jvi.4.5.688-693.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway E. G. Strategy of the flavivirus genome: evidence for multiple internal initiation of translation of proteins specified by Kunjin virus in mammalian cells. Virology. 1977 Jul 15;80(2):320–335. doi: 10.1016/s0042-6822(77)80008-1. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]


