Abstract
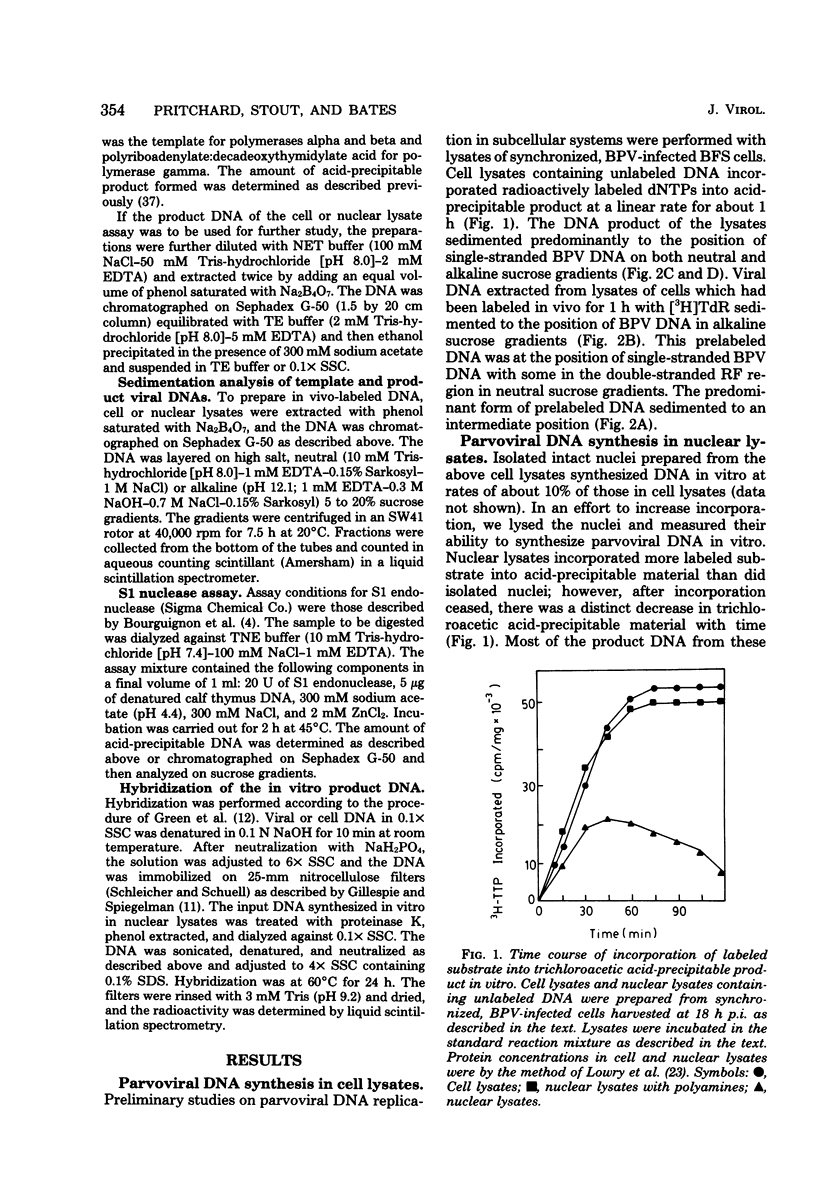
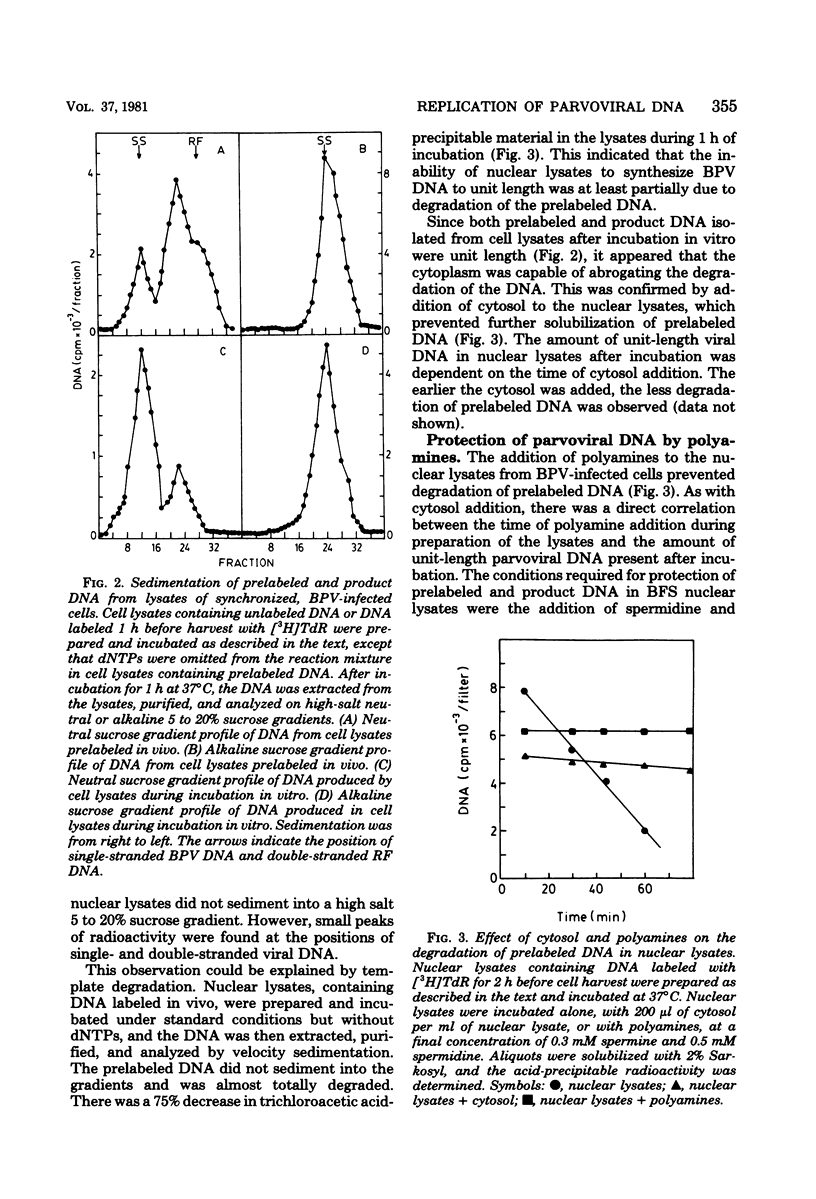
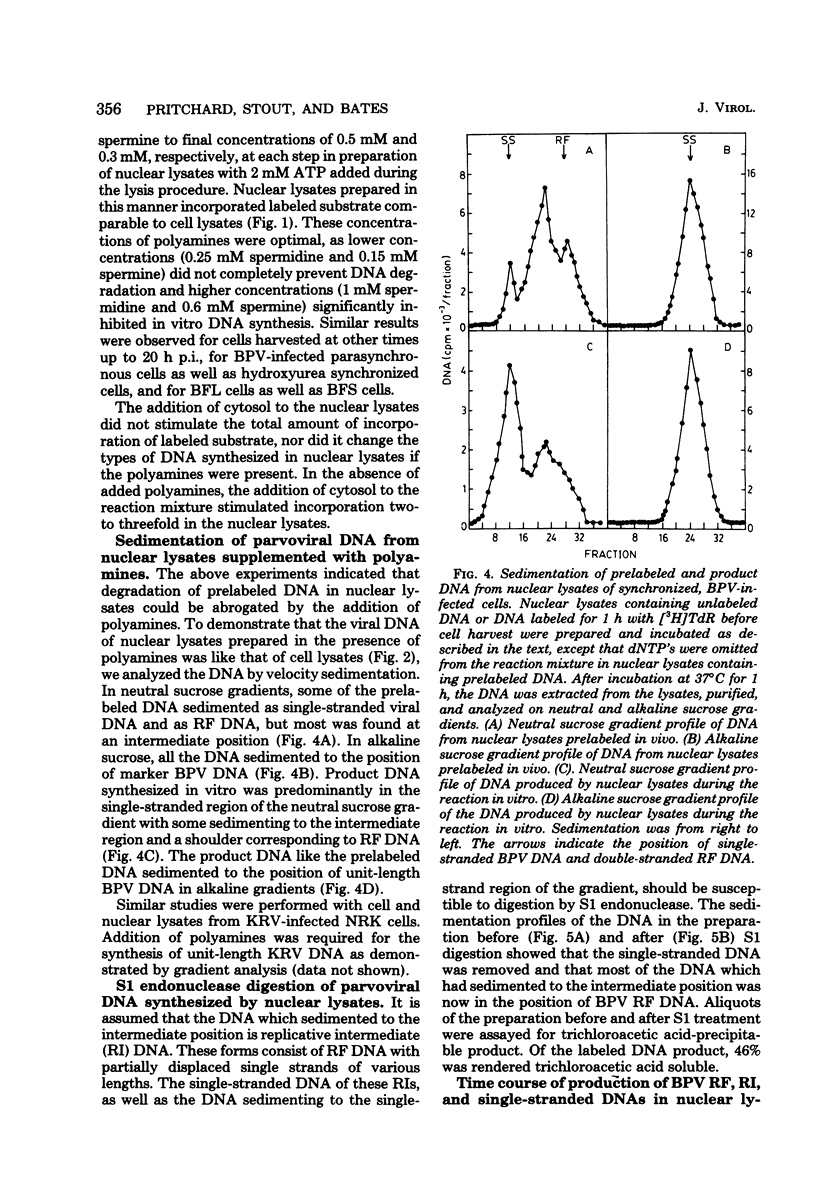
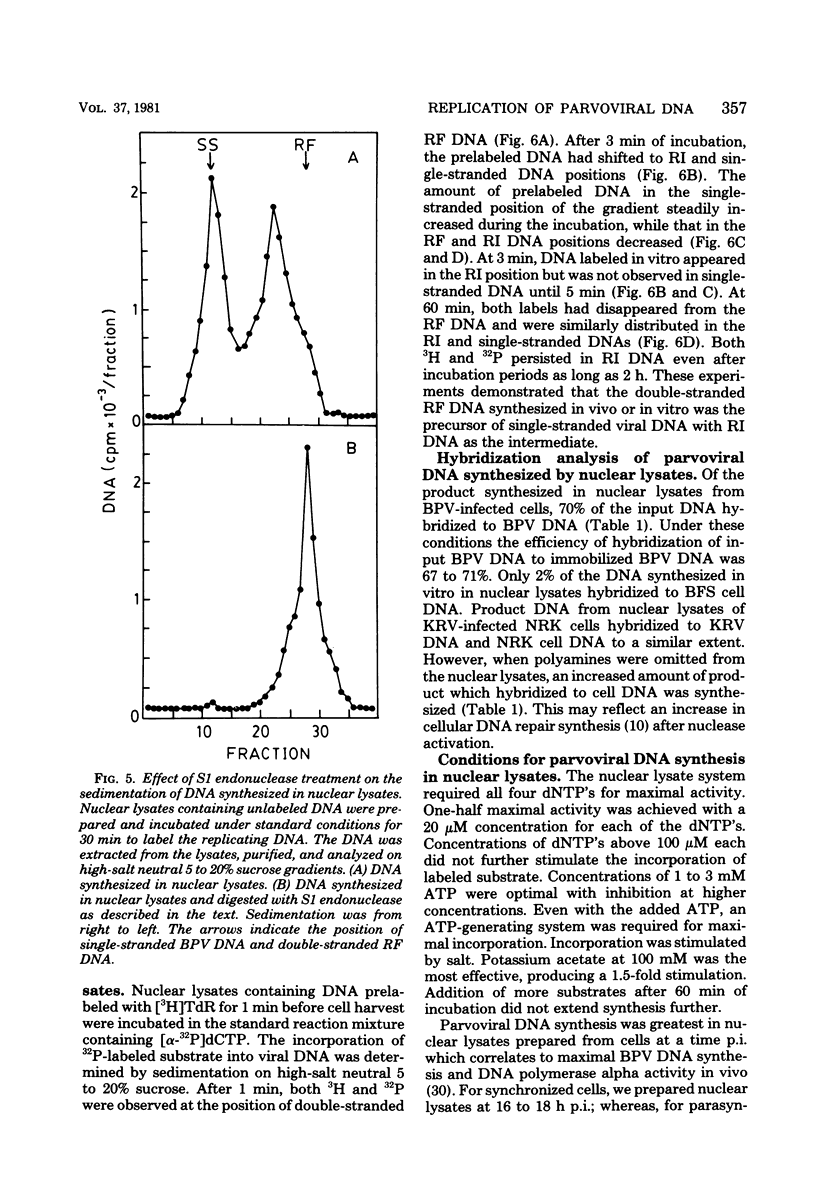
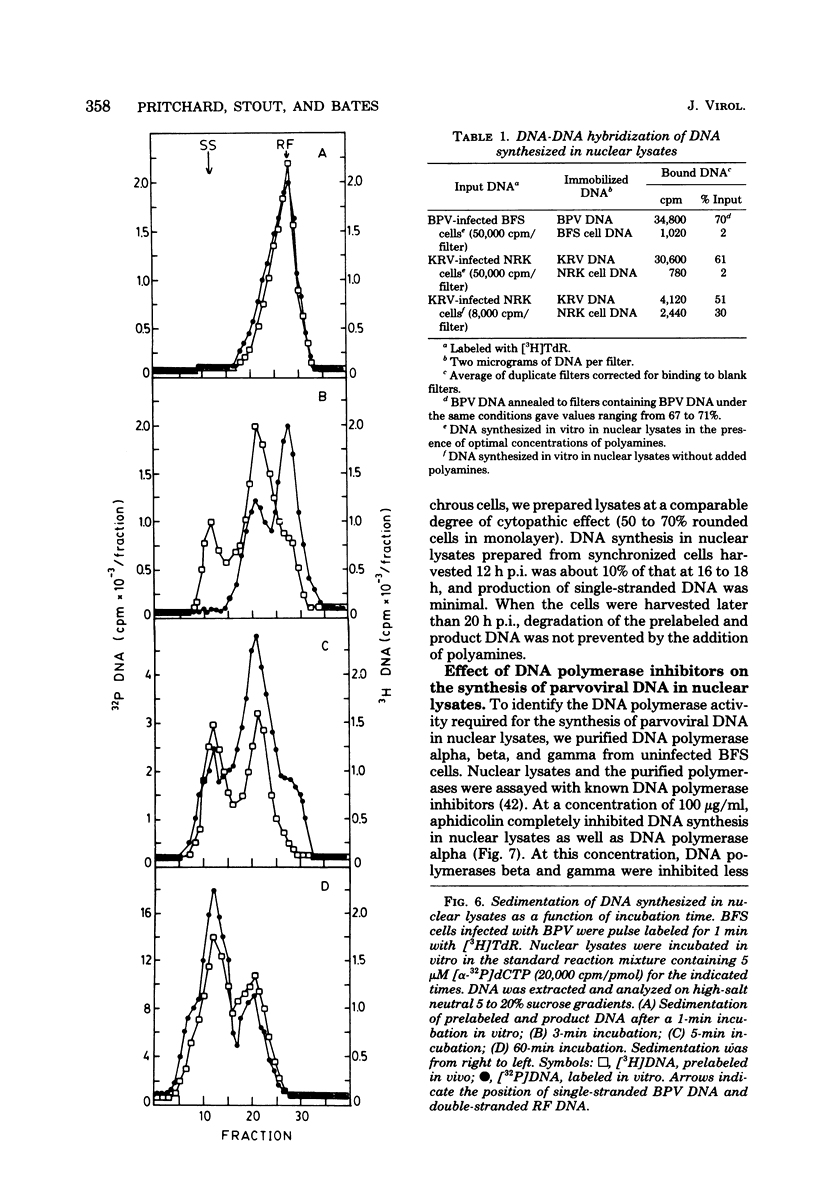
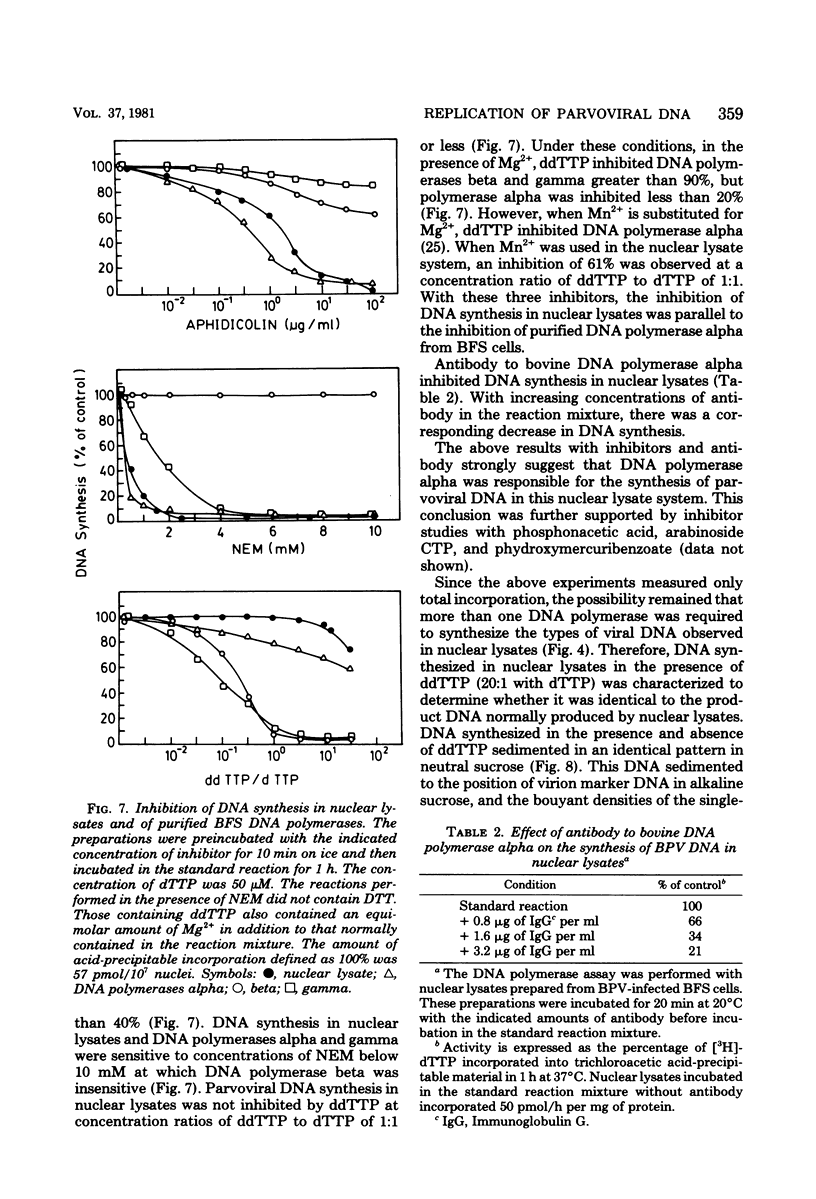
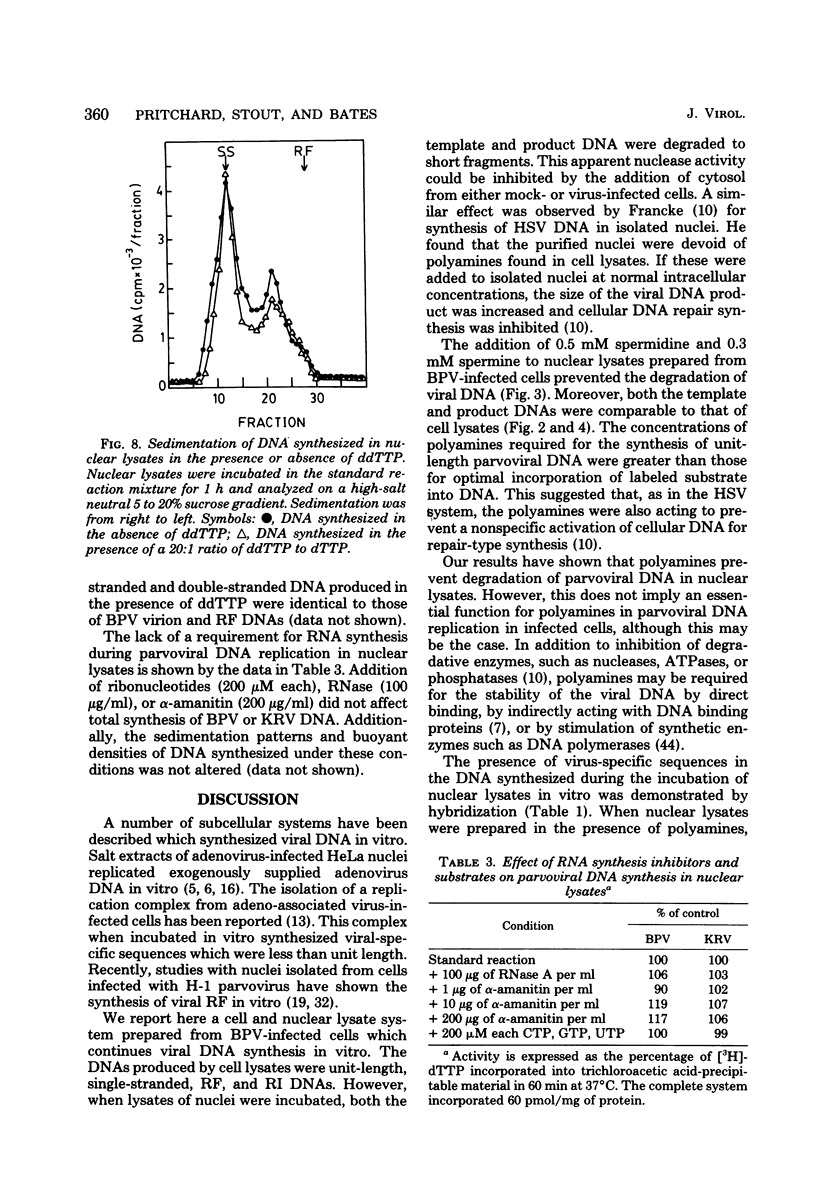
We have developed a nuclear lysate system from infected, synchronized cells capable of synthesizing unit-length parvoviral DNA in vitro. It was necessary to supplement the nuclear lysates with the polyamines, spermidine and spermine, to prevent degradation of template and product DNAs. In this system RF, RI, and single-stranded progeny DNAs were synthesized. Label incorporated in viral RF DNA in vivo appeared first in RI DNA and then in single-stranded DNA during incubation in vitro. By sedimentation the product DNAs were identical to those found in infected cells. Their viral identity was confirmed by hybridization. The addition of ribonucleotides, RNase, or alpha-amanitin did not affect parvoviral DNA synthesis in this system. The results with the specific inhibitors of mammalian DNA polymerases, aphidicolin, N-ethylmaleimide, and 2',3'-dideoxythymidine 5'-triphosphate indicated that DNA polymerase alpha was required for synthesis of parvoviral DNA in the nuclear lysates. This requirement was confirmed by experiments with antibody to bovine DNA polymerase alpha.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourguignon G. J., Tattersall P. J., Ward D. C. DNA of minute virus of mice: self-priming, nonpermuted, single-stranded genome with a 5'-terminal hairpin duplex. J Virol. 1976 Oct;20(1):290–306. doi: 10.1128/jvi.20.1.290-306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro: origin and direction of daughter strand synthesis. J Mol Biol. 1979 Dec 25;135(4):999–1012. doi: 10.1016/0022-2836(79)90524-2. [DOI] [PubMed] [Google Scholar]
- Christiansen C., Baldwin R. L. Catalysis of DNA reassociation by the Escherichia coli DNA binding protein: A polyamine-dependent reaction. J Mol Biol. 1977 Sep 25;115(3):441–454. doi: 10.1016/0022-2836(77)90164-4. [DOI] [PubMed] [Google Scholar]
- Daugharty H., Warfield D. T., Nemingway N. D., Casey H. L. Mumps class-specific immunoglobulins in radioimmunoassay and conventional serology. Infect Immun. 1973 Mar;7(3):398–402. doi: 10.1128/iai.7.3.398-402.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J., Anderson S., DePamphilis M. L. Involvement of DNA polymerase alpha in simian virus 40 DNA replication. J Biol Chem. 1978 May 10;253(9):3273–3280. [PubMed] [Google Scholar]
- Francke B. Cell-free synthesis of herpes simplex virus DNA: the influence of polyamines. Biochemistry. 1978 Dec 12;17(25):5494–5499. doi: 10.1021/bi00618a026. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Handa H., Carter B. J. Adeno-associated virus DNA replication complexes in herpes simplex virus or adenovirus-infected cells. J Biol Chem. 1979 Jul 25;254(14):6603–6610. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Kaplan L. M., Ariga H., Hurwitz J., Horwitz M. S. Complementation of the temperature-sensitive defect in H5ts125 adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5534–5538. doi: 10.1073/pnas.76.11.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf K. W. Synthesis of herpes simplex virus DNA in isolated chromatin. Biochemistry. 1979 May 1;18(9):1776–1781. doi: 10.1021/bi00576a022. [DOI] [PubMed] [Google Scholar]
- Koczot F. J., Carter B. J., Garon C. F., Rose J. A. Self-complementarity of terminal sequences within plus or minus strands of adenovirus-associated virus DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):215–219. doi: 10.1073/pnas.70.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokan H., Schaffer P., DePamphilis M. L. Involvement of eucaryotic deoxyribonucleic acid polymerases alpha and gamma in the replication of cellular and viral deoxyribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4431–4443. doi: 10.1021/bi00587a025. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavelle G., Li A. T. Isolation of intracellular replicative forms and progeny single strands of DNA from parvovirus KRV in sucrose gradients containing guanidine hydrochloride. Virology. 1977 Jan;76(1):464–467. doi: 10.1016/0042-6822(77)90324-5. [DOI] [PubMed] [Google Scholar]
- Ono K., Ogasawara M., Matsukage A. Inhibition of the activity of DNA polymerase alpha by 2',3'-dideoxythymidine 5'-triphosphate. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1255–1262. doi: 10.1016/0006-291x(79)91115-x. [DOI] [PubMed] [Google Scholar]
- Parris D. S., Bates R. C. Effect of bovine parvovirus replication on DNA, RNA, and protein synthesis in S phase cells. Virology. 1976 Aug;73(1):72–78. doi: 10.1016/0042-6822(76)90061-1. [DOI] [PubMed] [Google Scholar]
- Parris D. S., Bates R. C., Stout E. R. Hydroxyurea synchoronization of bovine fetal spleen cells. Exp Cell Res. 1975 Dec;96(2):422–425. doi: 10.1016/0014-4827(75)90278-5. [DOI] [PubMed] [Google Scholar]
- Patton J. T., Stout E. R., Bates R. C. Transcription of the bovine parvovirus genome in isolated nuclei. J Virol. 1979 Jun;30(3):917–922. doi: 10.1128/jvi.30.3.917-922.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Huberman J. A. Asymmetric Okazaki piece synthesis during replication of simian virus 40 DNA in vivo. Cell. 1977 Dec;12(4):1029–1043. doi: 10.1016/0092-8674(77)90167-2. [DOI] [PubMed] [Google Scholar]
- Pritchard C., Bates R. C., Stout E. R. Levels of cellular DNA polymerases in synchronized bovine paravovirus-infected cells. J Virol. 1978 Jul;27(1):258–261. [PMC free article] [PubMed] [Google Scholar]
- Pritchard C., Patton J. T., Bates R. C., Stout E. R. Replication of nondefective parvoviruses: lack of a virion-associated DNA polymerase. J Virol. 1978 Oct;28(1):20–27. doi: 10.1128/jvi.28.1.20-27.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. VI. Characterization of a replication terminus of H-1 replicative-form DNA. J Virol. 1977 Feb;21(2):694–712. doi: 10.1128/jvi.21.2.694-712.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A. Evidence for terminal S1-nuclease-resistant regions on single-stranded linear DNA. Virology. 1977 Jan;76(1):454–458. doi: 10.1016/0042-6822(77)90322-1. [DOI] [PubMed] [Google Scholar]
- Siegl G., Gautschi M. The multiplication of parvovirus Lu3 in a synchronized culture system. I. Optimum conditions for virus replication. Arch Gesamte Virusforsch. 1973;40(1):105–118. doi: 10.1007/BF01242642. [DOI] [PubMed] [Google Scholar]
- Stout E. R., Arens M. Q. DNA polymerase from maize seedlings. Biochim Biophys Acta. 1970 Jul 16;213(1):90–100. doi: 10.1016/0005-2787(70)90010-9. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P. Replication of the parvovirus MVM. I. Dependence of virus multiplication and plaque formation on cell growth. J Virol. 1972 Oct;10(4):586–590. doi: 10.1128/jvi.10.4.586-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Hand R. E., Jr Requirement of cellular synthesis for Kilham rat virus replication. Virology. 1970 Dec;42(4):1054–1063. doi: 10.1016/0042-6822(70)90353-3. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Layman K. R., Hand R. E. Effect of cell physiological state on infection by rat virus. J Virol. 1969 Dec;4(6):872–878. doi: 10.1128/jvi.4.6.872-878.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach A. Vertebrate DNA polymerases. Cell. 1975 Jun;5(2):101–108. doi: 10.1016/0092-8674(75)90017-3. [DOI] [PubMed] [Google Scholar]
- Yamada M., Brun G., Weissbach A. Synthesis of viral and host DNA in isolated chromatin from herpes simplex virus-infected HeLa cells. J Virol. 1978 May;26(2):281–290. doi: 10.1128/jvi.26.2.281-290.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Masaki S., Ando T. Effects of polyamines on in vitro dna synthesis by DNA polymerases from calf thymus. J Biochem. 1976 May;79(5):895–901. doi: 10.1093/oxfordjournals.jbchem.a131157. [DOI] [PubMed] [Google Scholar]


