Abstract
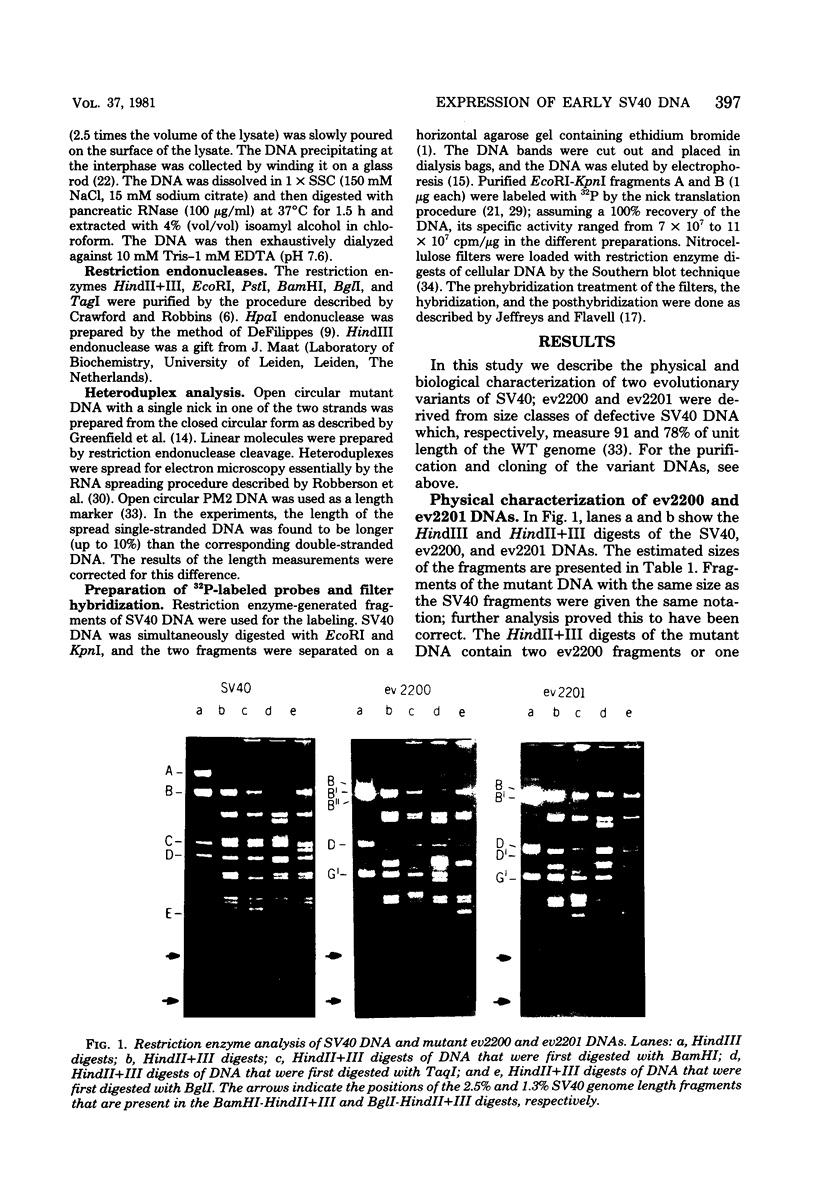
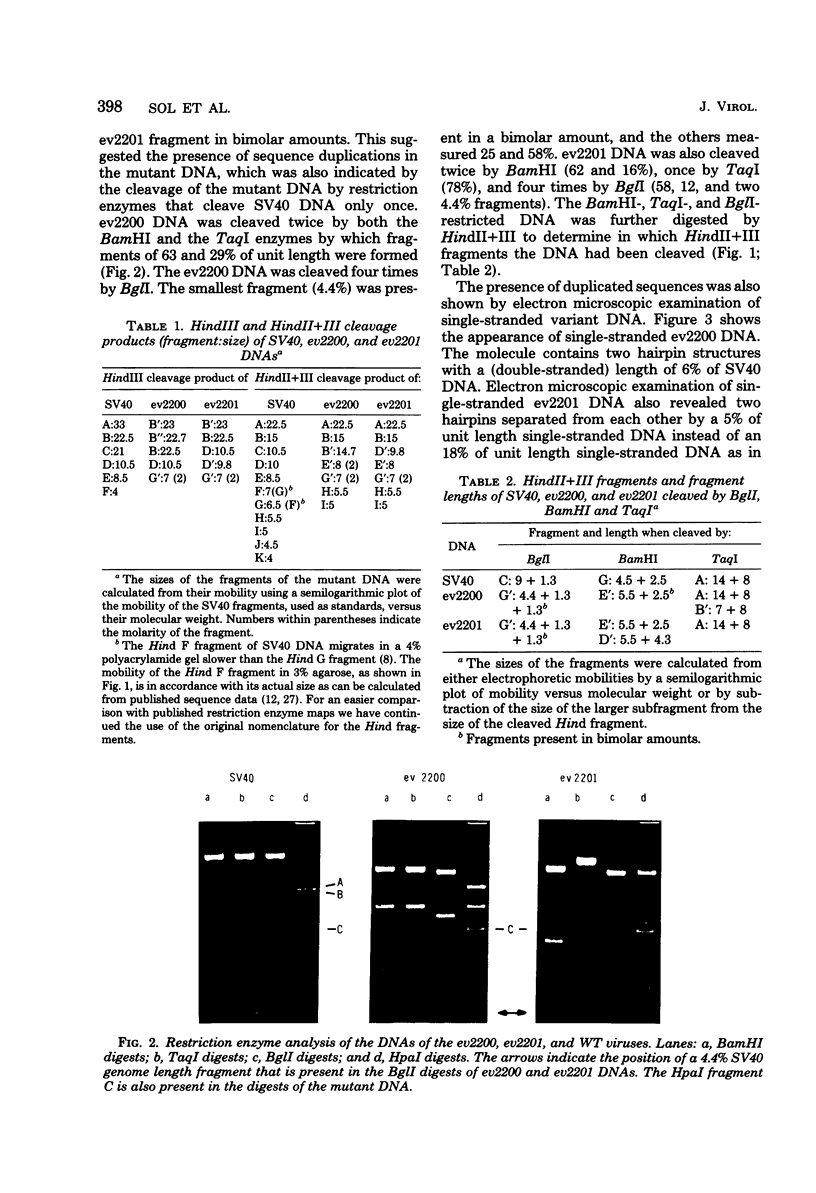
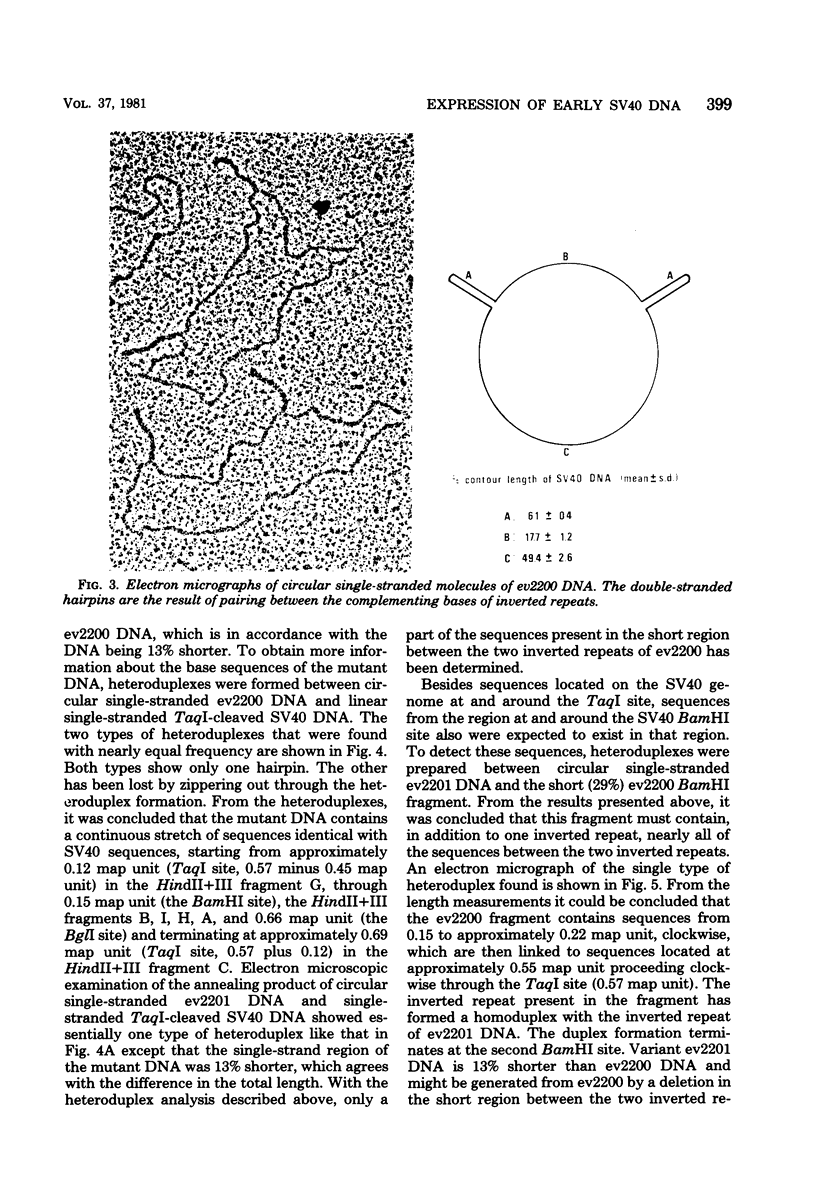
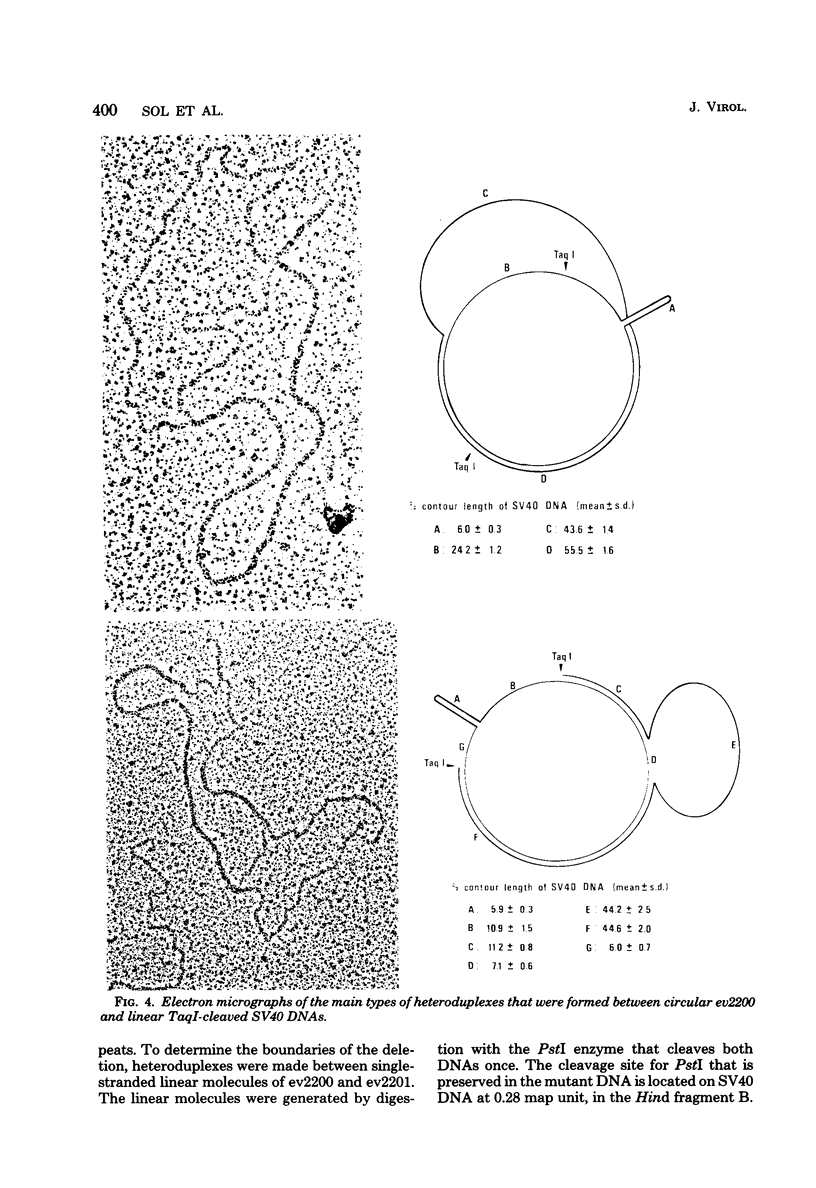
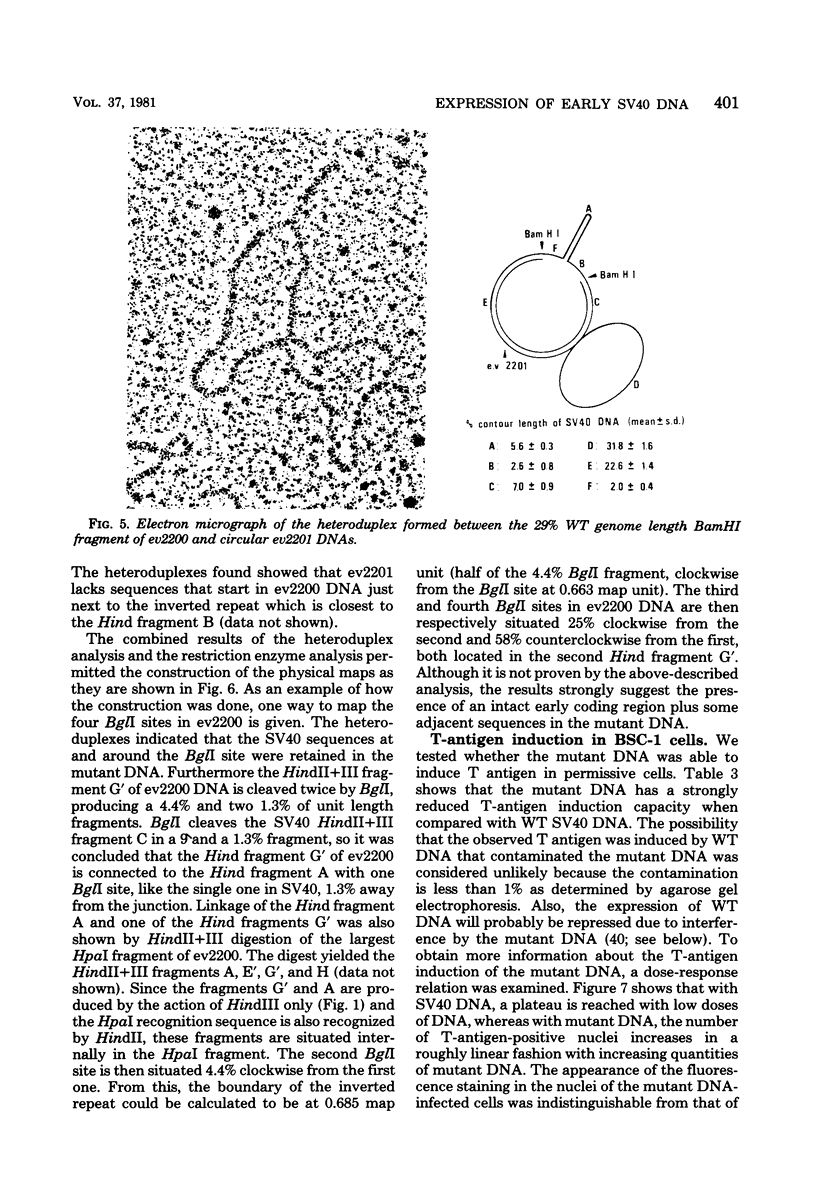
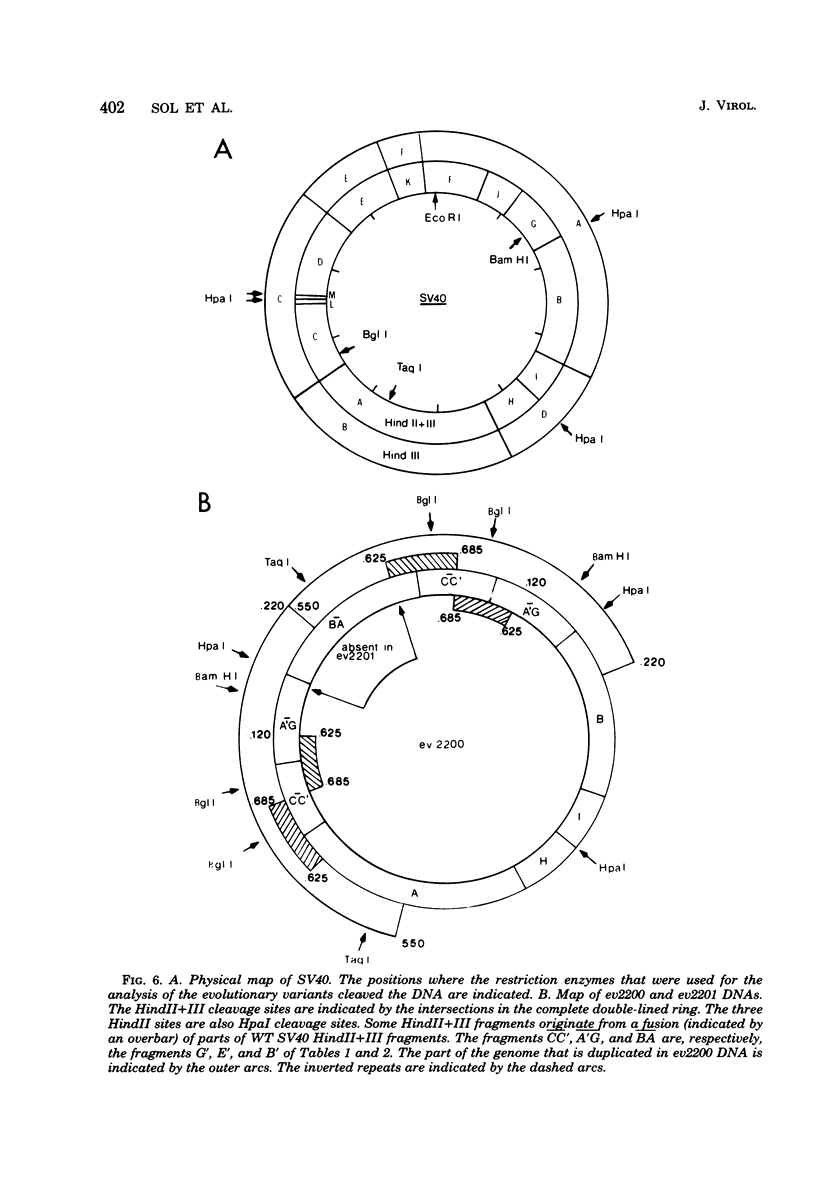
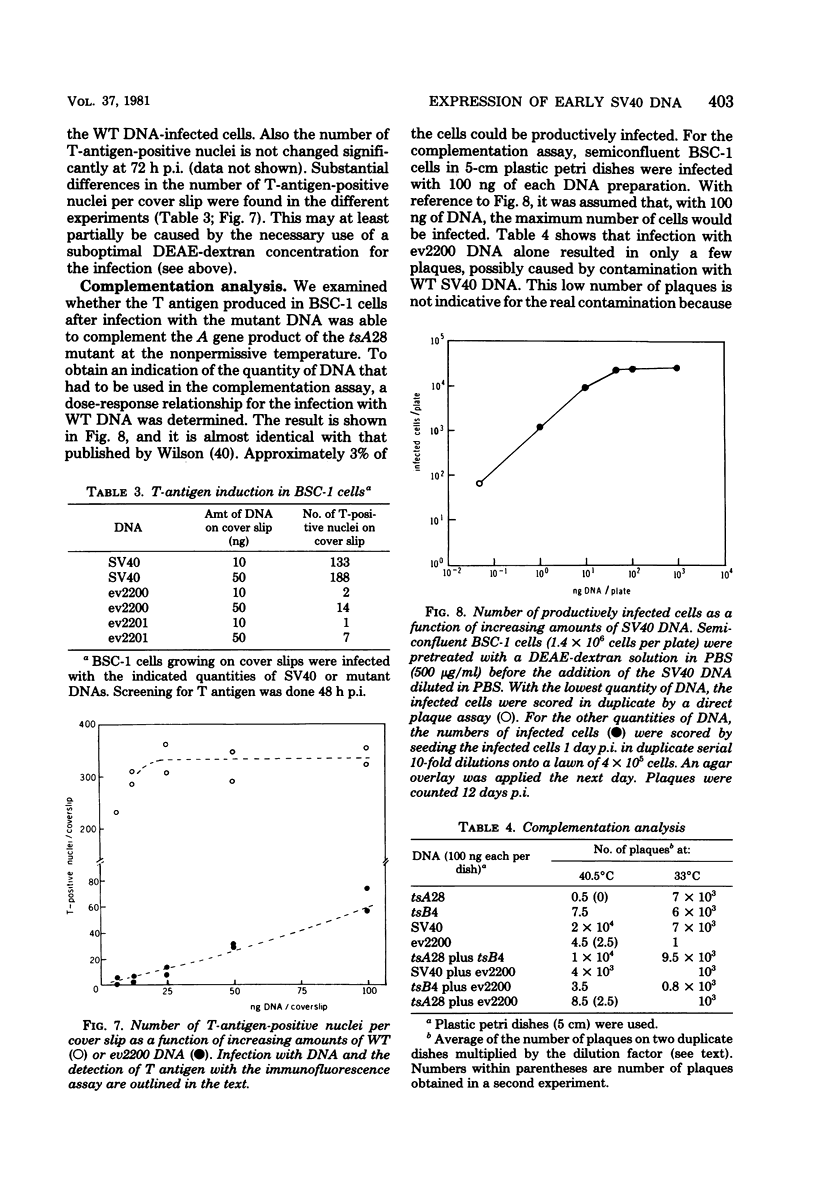
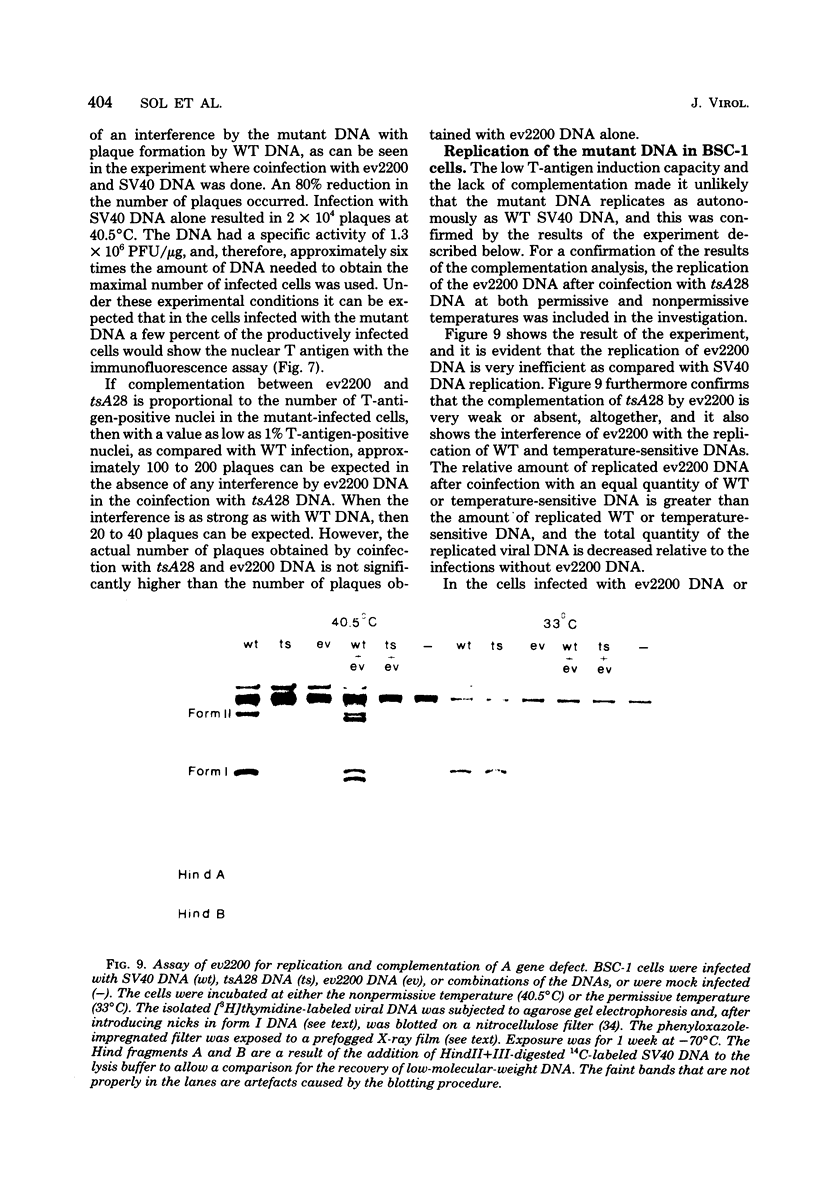
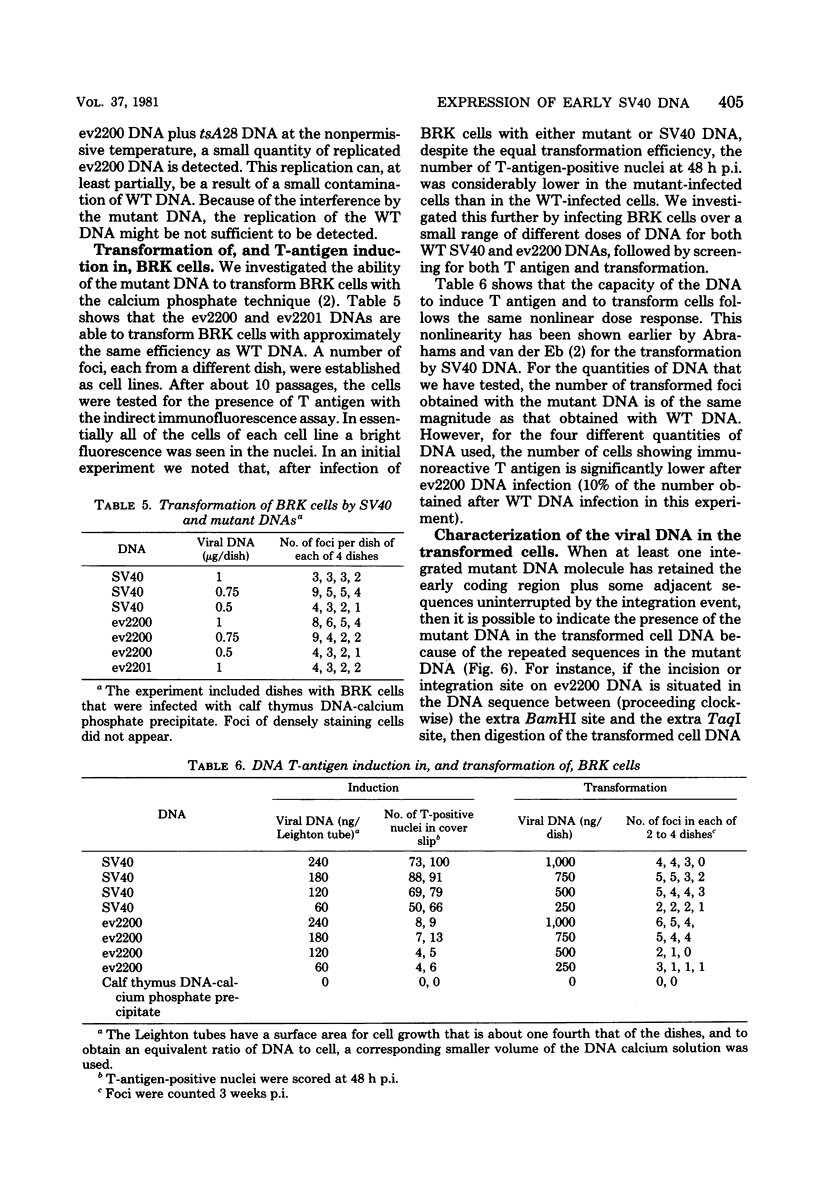
From an undiluted passaged virus stock, two size classes of defective simian virus 40 (SV40) DNA were isolated from which two evolutionary variants were cloned. By means of restriction enzyme and heteroduplex analysis, physical maps of the mutants have been constructed. Both mutants contained the region of SV40 DNA coding for the early proteins plus some adjacent sequences (the region from 0.120 to 0.685 map unit, clockwise, on the standard SV40 DNA map). Furthermore, each mutant contained, in the form of two inverted repeats, four times the sequences from the region 0.625 to 0.685 map unit, clockwise. Some biological properties of the mutant DNA were examined, and we found that the mutant DNA (i) has, as compared with SV40 DNA, an impaired ability to induce T antigen in permissive and nonpermissive cells; (ii) does not complement a thermosensitive A mutant of SV40; (iii) replicates very inefficiently without a helper; and (iv), as an apparent contradiction, transforms nonpermissive baby rat kidney cells as well as SV40 DNA. A hypothetical mechanism for the expression of the mutant DNA that might explain the observed biological properties is presented.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaij C., Borst P. The gel electrophoresis of DNA. Biochim Biophys Acta. 1972 May 10;269(2):192–200. doi: 10.1016/0005-2787(72)90426-1. [DOI] [PubMed] [Google Scholar]
- Abrahams P. J., Van der Eb A. J. In vitro transformation of rat and mouse cells by DNA from simian virus 40. J Virol. 1975 Jul;16(1):206–209. doi: 10.1128/jvi.16.1.206-209.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Landers T., Goff S. P., Manteuil-Brutlag S., Berg P. Physical and genetic characterization of deletion mutants of simian virus 40 constructed in vitro. J Virol. 1977 Oct;24(1):277–294. doi: 10.1128/jvi.24.1.277-294.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Robbins A. K. The cleavage of polyoma virus DNA by restriction enzymes KpnI and PstI. J Gen Virol. 1976 Jun;31(3):315–321. doi: 10.1099/0022-1317-31-3-315. [DOI] [PubMed] [Google Scholar]
- Danna K. J., Nathans D. Bidirectional replication of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3097–3100. doi: 10.1073/pnas.69.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Sack G. H., Jr, Nathans D. Studies of simian virus 40 DNA. VII. A cleavage map of the SV40 genome. J Mol Biol. 1973 Aug 5;78(2):363–376. doi: 10.1016/0022-2836(73)90122-8. [DOI] [PubMed] [Google Scholar]
- DeFilippes F. M. A new method for isolation of a restriction enzyme from Hemophilus parainfluenzae. Biochem Biophys Res Commun. 1974 Jun 4;58(3):586–596. doi: 10.1016/s0006-291x(74)80460-2. [DOI] [PubMed] [Google Scholar]
- Dhar R., Subramanian K. N., Pan J., Weissman S. M. Nucleotide sequence of a fragment of SV40 DNA that contains the origin of DNA replication and specifies the 5' ends of "early" and "late" viral RNA. IV. Localization of the SV40 DNA complementary to the 5' ends of viral mRNA. J Biol Chem. 1977 Jan 10;252(1):368–376. [PubMed] [Google Scholar]
- Fareed G. C., Garon G. F., Salzman N. P. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):484–491. doi: 10.1128/jvi.10.3.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Fried M. Isolation and partial characterization of different defective DNA molecules derived from polyoma virus. J Virol. 1974 May;13(5):939–946. doi: 10.1128/jvi.13.5.939-946.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield L., Simpson L., Kaplan D. Conversion of closed circular DNA molecules to single-nicked molecules by digestion with DNAase I in the presence of ethidium bromide. Biochim Biophys Acta. 1975 Oct 15;407(3):365–375. doi: 10.1016/0005-2787(75)90104-5. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Khoury G., May E. Regulation of early and late simian virus 40 transcription: overproduction of early viral RNA in the absence of a functional T-antigen. J Virol. 1977 Jul;23(1):167–176. doi: 10.1128/jvi.23.1.167-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lee T. N., Nathans D. A transcriptional map of the SV40 genome in transformed cell lines. Virology. 1975 Jan;63(1):263–272. doi: 10.1016/0042-6822(75)90390-6. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Ozanne B., Sharp P. A., Sambrook J. Transcription of simian virus 40. II. Hybridization of RNA extracted from different lines of transformed cells to the separated strands of simian virus 40 DNA. J Virol. 1973 Jul;12(1):90–98. doi: 10.1128/jvi.12.1.90-98.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Stark G. R., Alwine J. C. Autoregulation of simian virus 40 gene A by T antigen. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3083–3087. doi: 10.1073/pnas.73.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robberson D., Aloni Y., Attardi G., Davidson N. Expression of the mitochondrial genome in HeLa cells. VI. Size determination of mitochondrial ribosomal RNA by electron microscopy. J Mol Biol. 1971 Sep 28;60(3):473–484. doi: 10.1016/0022-2836(71)90182-3. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. A biochemical method for increasing the size of deletion mutations in simian virus 40 DNA. J Mol Biol. 1977 Jul 5;113(3):503–515. doi: 10.1016/0022-2836(77)90235-2. [DOI] [PubMed] [Google Scholar]
- Sol C. J., Walig C., ter Schegget J., van der Noordaa J. Analysis of defective SV40 DNA by agarose gel electrophoresis. J Gen Virol. 1975 Sep;28(3):285–297. doi: 10.1099/0022-1317-28-3-285. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N. Segments of simian virus 40 DNA spanning most of the leader sequence of the major late viral messenger RNA are dispensable. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2556–2560. doi: 10.1073/pnas.76.6.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Van Heuverswyn H., Fiers W. Nucleotide sequence of the Hind-C fragment of simian virus 40 DNA. Comparison of the 5'-untranslated region of wild-type virus and of some deletion Mutants. Eur J Biochem. 1979 Oct;100(1):51–60. doi: 10.1111/j.1432-1033.1979.tb02032.x. [DOI] [PubMed] [Google Scholar]
- Wall R., Darnell J. E. Presence of cell and virus specific sequences in the same molecules of nuclear RNA from virus transformed cells. Nat New Biol. 1971 Jul 21;232(29):73–76. doi: 10.1038/newbio232073a0. [DOI] [PubMed] [Google Scholar]
- Wilson J. H. Interference in SV40 DNA infections: a possible basis for cellular competence. Virology. 1978 Dec;91(2):380–388. doi: 10.1016/0042-6822(78)90385-9. [DOI] [PubMed] [Google Scholar]