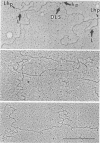
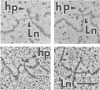
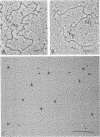
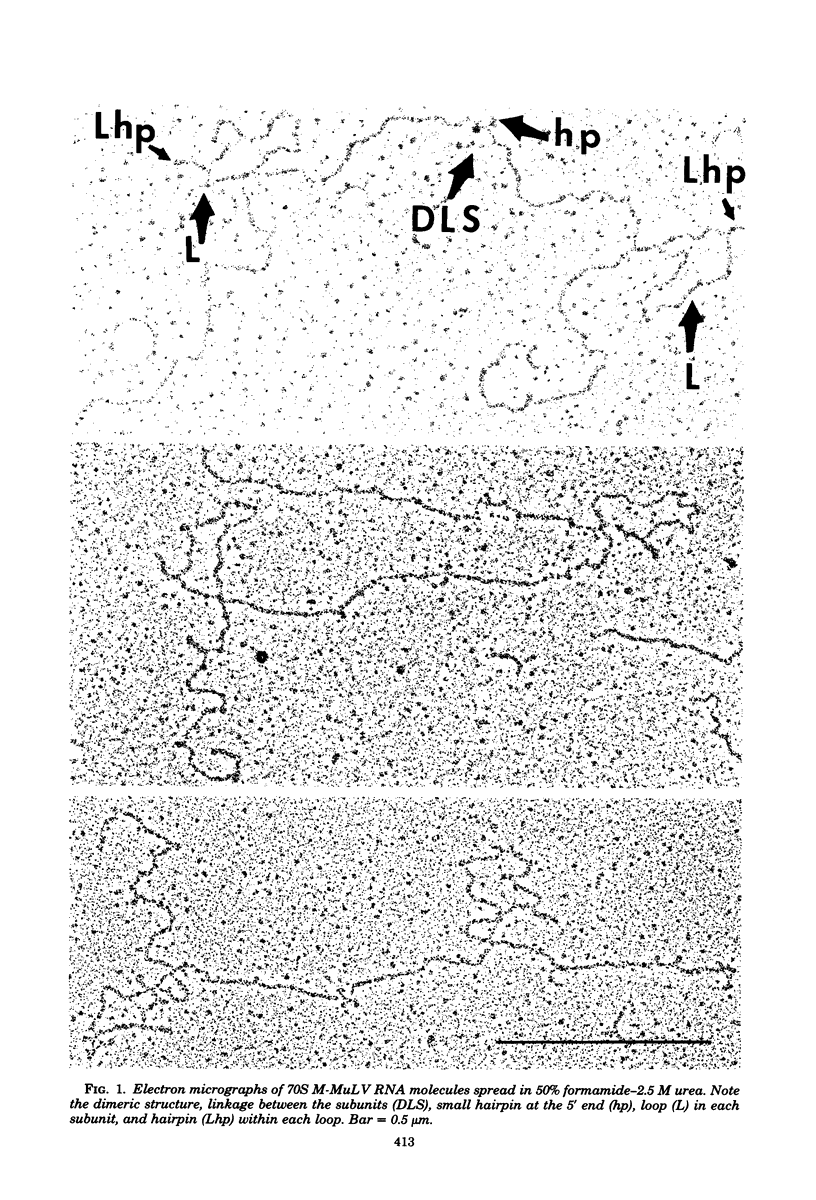
Abstract
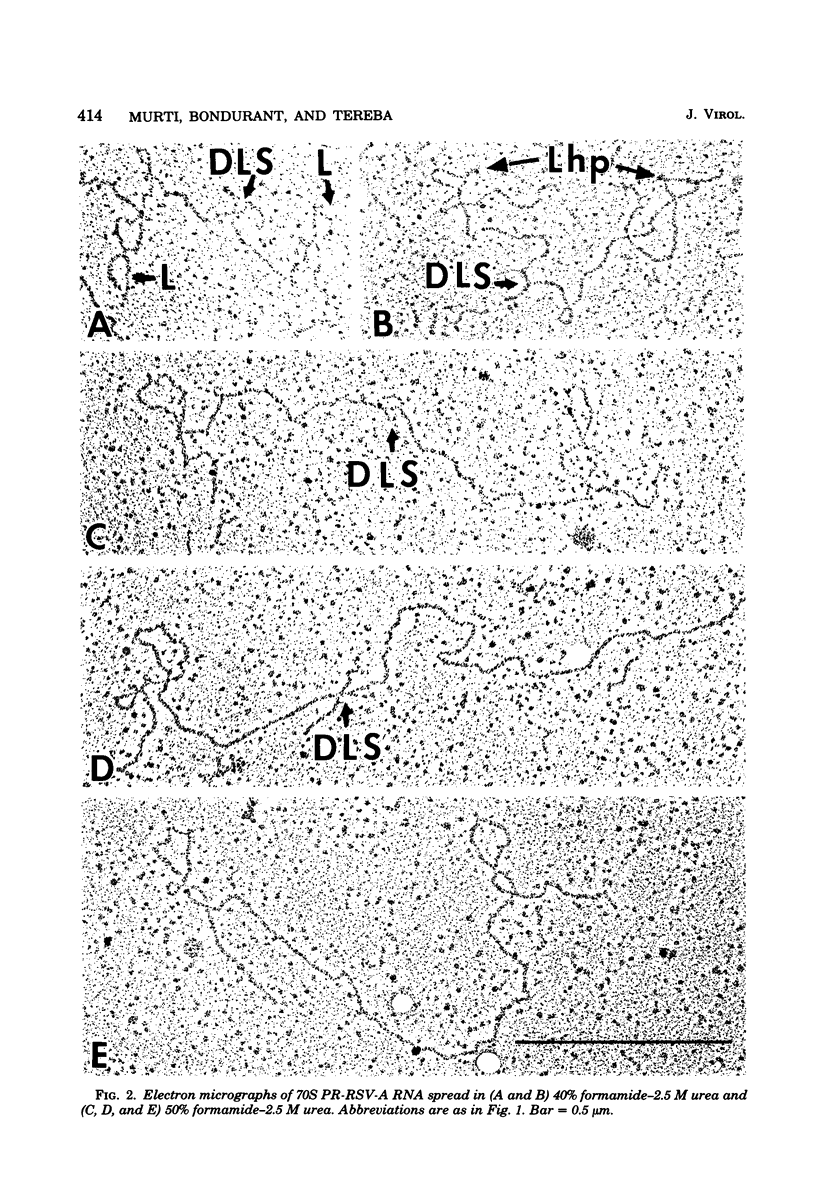
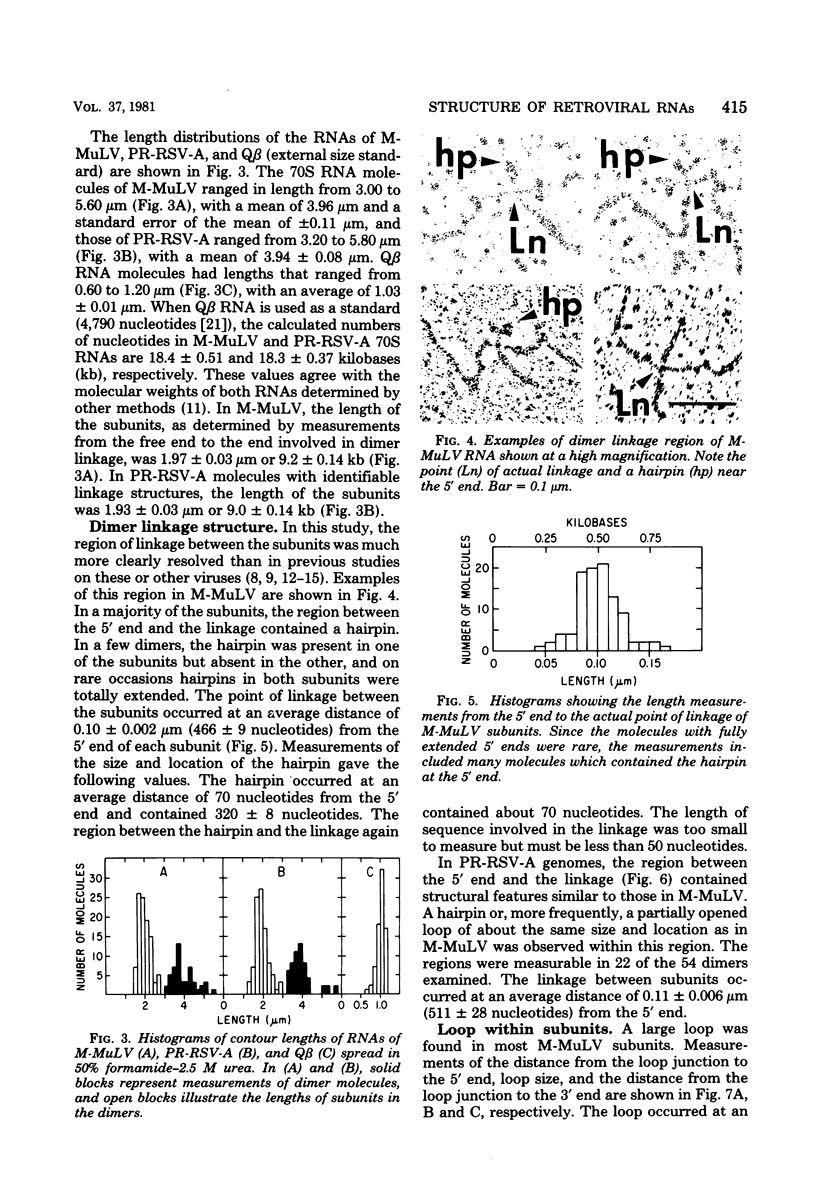
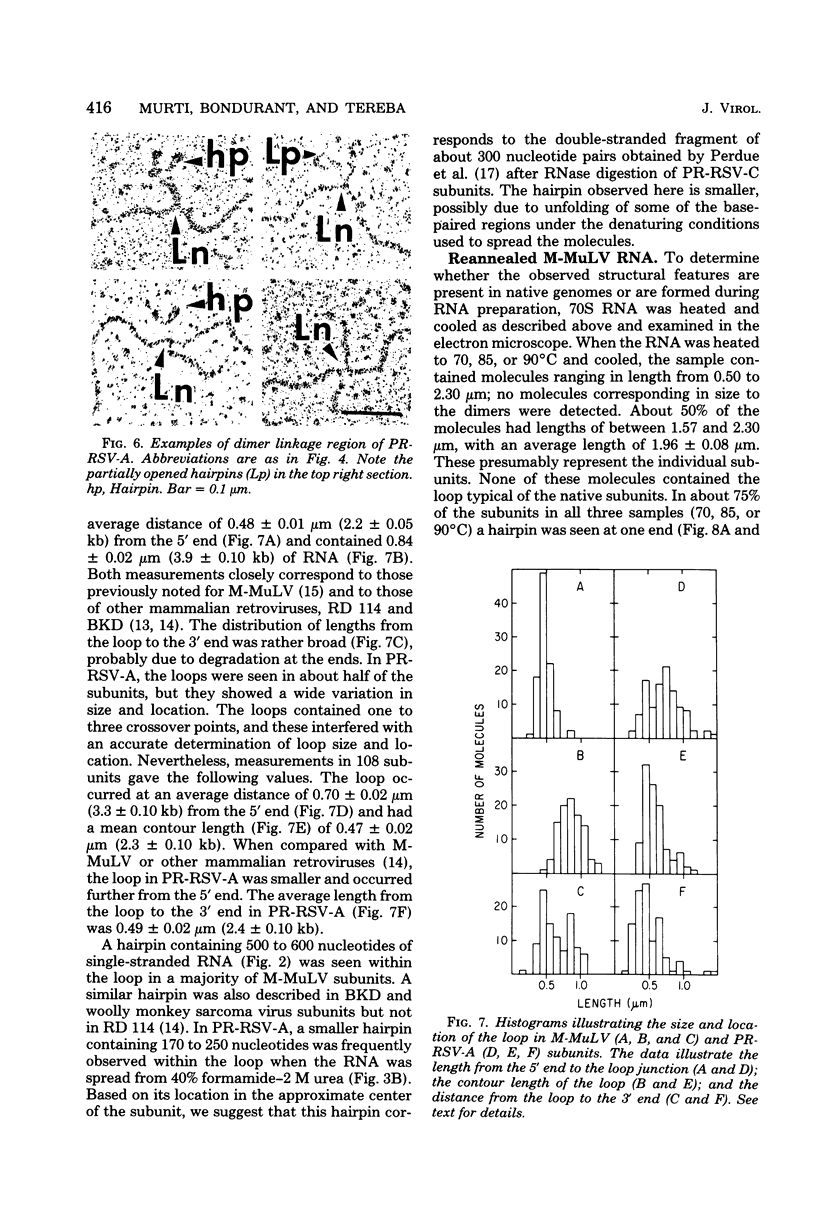
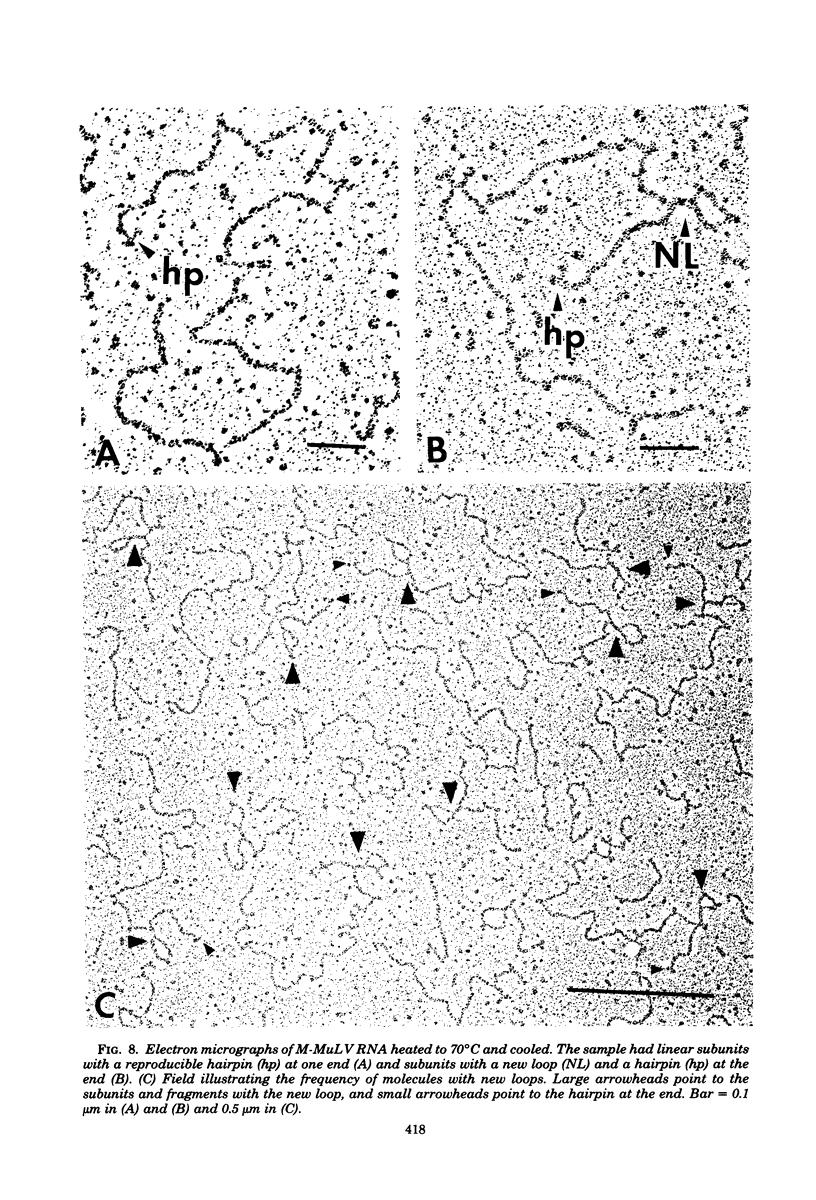
The secondary structural features in the 70S RNAs of the Prague strain of avian Rous sarcoma virus, subgroup A (PR-RSV-A), and Moloney murine leukemia virus (M-MuLV) were compared by electron microscopy. The PR-RSV-A genome contained two subunits joined by a linkage structure as in the genomes of M-MuLV and other mammalian retroviruses. In both viral genomes, a highly reproducible hairpin occurred at about 70 nucleotides from the 5' end of each subunit and contained 320 +/- 8 nucleotides. The stable point of linkage between the subunits in both viral genomes involved fewer than 50 nucleotides and occurred at 466 +/- 9 nucleotides from the 5' end. This places the linkage about 350 nucleotides further toward the 3' end of the subunit than the binding site of primer tRNA. Another structural feature common to both genomes was a loop in each subunit. In M-MuLV, the loop contained 3.9 +/- 0.10 kilobases (kb) and occurred at a distance of 2.2 +/- 0.05 kb from the 5' end. In PR-RSV-A, the loop was smaller (2.3 +/- 0.10 kb) and further (3.3 +/- 0.10 kb) from the 5' end. When M-MuLV RNA was heated to 70, 85, or 90 degrees C and cooled, the hairpin consistently reformed at the 5' end. No other structures typical of the native molecules reappeared. In RNA samples heated to 70 degrees C, a new loop reproducibly occurred near the 5' end of each subunit, but this loop was not found in samples heated to higher temperatures. Based on all of these findings, we conclude that the genome of PR-RSV-A shares several features with M-MuLV and other mammalian retroviruses and that the primer tRNA molecules are not involved in the linkage of the two subunits in either genome. We also conclude that the dimer linkage and the loops in subunits are typical of the native molecules and that their formation requires a special environment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Ray D. A. Configurational variants of oncornavirus RNAs. J Virol. 1976 Sep;19(3):810–819. doi: 10.1128/jvi.19.3.810-819.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender W., Davidson N. Mapping of poly(A) sequences in the electron microscope reveals unusual structure of type C oncornavirus RNA molecules. Cell. 1976 Apr;7(4):595–607. doi: 10.1016/0092-8674(76)90210-5. [DOI] [PubMed] [Google Scholar]
- Bronson D. L., Elliott A. Y., Ritzi D. A comparison of four methods used to concentrate Rous sarcoma virus from tissue culture fluids. J Gen Virol. 1976 Dec;33(3):403–410. doi: 10.1099/0022-1317-33-3-403. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Hageman T. C., Maxam A. M., Haseltine W. A. Structure of the genome of Moloney murine leukemia virus: a terminally redundant sequence. Cell. 1978 Apr;13(4):761–773. doi: 10.1016/0092-8674(78)90226-x. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Darlix J. L., Spahr P. F., Bromley P. A. Analysis of Rous sarcoma virus (RSV) RNA structure by means of specific nucleases. Virology. 1978 Oct 15;90(2):317–329. doi: 10.1016/0042-6822(78)90316-1. [DOI] [PubMed] [Google Scholar]
- Delius H., Duesberg P. H., Mangel W. F. Electron microscope measurements of rous sarcoma virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):835–843. doi: 10.1101/sqb.1974.039.01.097. [DOI] [PubMed] [Google Scholar]
- Dube S., Kung H. J., Bender W., Davidson N., Ostertag W. Size, subunit composition, and secondary structure of the Friend virus genome. J Virol. 1976 Oct;20(1):264–272. doi: 10.1128/jvi.20.1.264-272.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda M. A., Rice N. R., Gilden R. V. Avian reticuloendotheliosis virus: characterization of the high-molecular-weight viral RNA in transforming and helper virus populations. J Virol. 1980 Jun;34(3):743–751. doi: 10.1128/jvi.34.3.743-751.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. M. High molecular weight RNAs from Rous sarcoma virus and Moloney murine leukemia virus contain two subunits. J Biol Chem. 1976 Jan 10;251(1):141–149. [PubMed] [Google Scholar]
- Kung H. J., Bailey J. M., Davidson N., Nicolson M. O., McAllister R. M. Structure, subunit composition, and molecular weight of RD-114 RNA. J Virol. 1975 Aug;16(2):397–411. doi: 10.1128/jvi.16.2.397-411.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Bailey J. M., Davidson N., Vogt P. K., Nicolson M. O., McAllister R. M. Electron microscope studies of tumor virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):827–834. doi: 10.1101/sqb.1974.039.01.096. [DOI] [PubMed] [Google Scholar]
- Kung H. J., Hu S., Bender W., Bailey J. M., Davidson N., Nicolson M. O., McAllister R. M. RD-114, baboon, and woolly monkey viral RNA's compared in size and structure. Cell. 1976 Apr;7(4):609–620. doi: 10.1016/0092-8674(76)90211-7. [DOI] [PubMed] [Google Scholar]
- Maisel J., Bender W., Hu S., Duesberg P. H., Davidson N. Structure of 50 to 70S RNA from Moloney sarcoma viruses. J Virol. 1978 Jan;25(1):384–394. doi: 10.1128/jvi.25.1.384-394.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Perdue M. L., Wunderli W., Joklik W. K. Isolation and characterization of a large "hairpin" segment from avian retrovirus RNA. Virology. 1979 May;95(1):24–35. doi: 10.1016/0042-6822(79)90398-2. [DOI] [PubMed] [Google Scholar]
- Riggin C. H., Bondurant M., Mitchell W. M. Physical properties of moloney murine leukemia virus high-molecular-weight RNA: a two subunit structure. J Virol. 1975 Dec;16(6):1528–1535. doi: 10.1128/jvi.16.6.1528-1535.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D., Aloni Y., Attardi G., Davidson N. Expression of the mitochondrial genome in HeLa cells. VI. Size determination of mitochondrial ribosomal RNA by electron microscopy. J Mol Biol. 1971 Sep 28;60(3):473–484. doi: 10.1016/0022-2836(71)90182-3. [DOI] [PubMed] [Google Scholar]
- Stoltzfus C. M., Snyder P. N. Structure of B77 sarcoma virus RNA: stabilization of RNA after packaging. J Virol. 1975 Nov;16(5):1161–1170. doi: 10.1128/jvi.16.5.1161-1170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider H. J., Stettler U., Kübler O., Koller T., Weber H. Refined molecular weights for phage, viral and ribosomal RNA. Gene. 1978 Jul;3(4):353–357. doi: 10.1016/0378-1119(78)90044-6. [DOI] [PubMed] [Google Scholar]
- Weissmann C., Parsons J. T., Coffin J. W., Rymo L., Billeter M. A., Hofstetter H. Studies on the structure and synthesis of Rous sarcoma virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1043–1056. doi: 10.1101/sqb.1974.039.01.120. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of RNA: processing of HeLa ribosomal RNA. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2827–2831. doi: 10.1073/pnas.70.10.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]