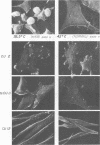
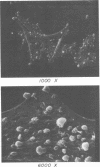
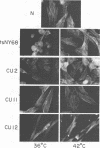
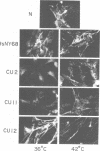
Abstract
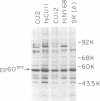
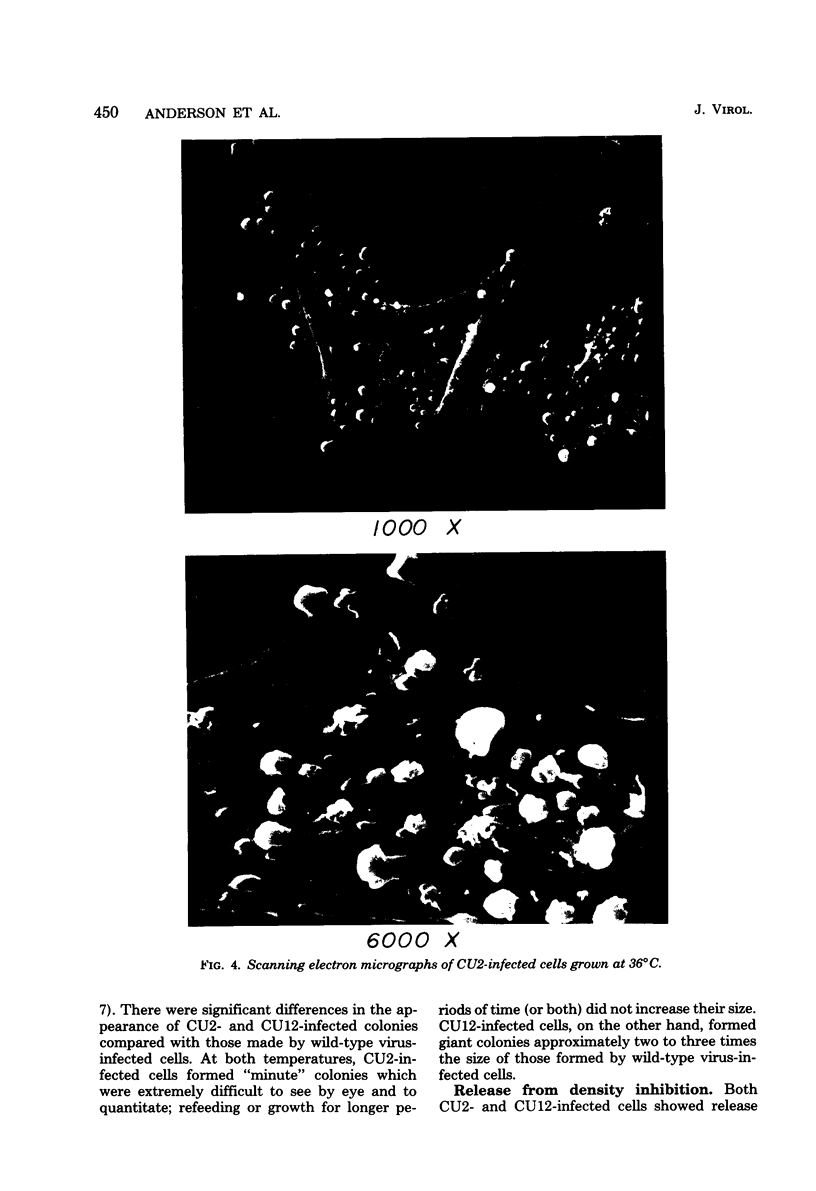
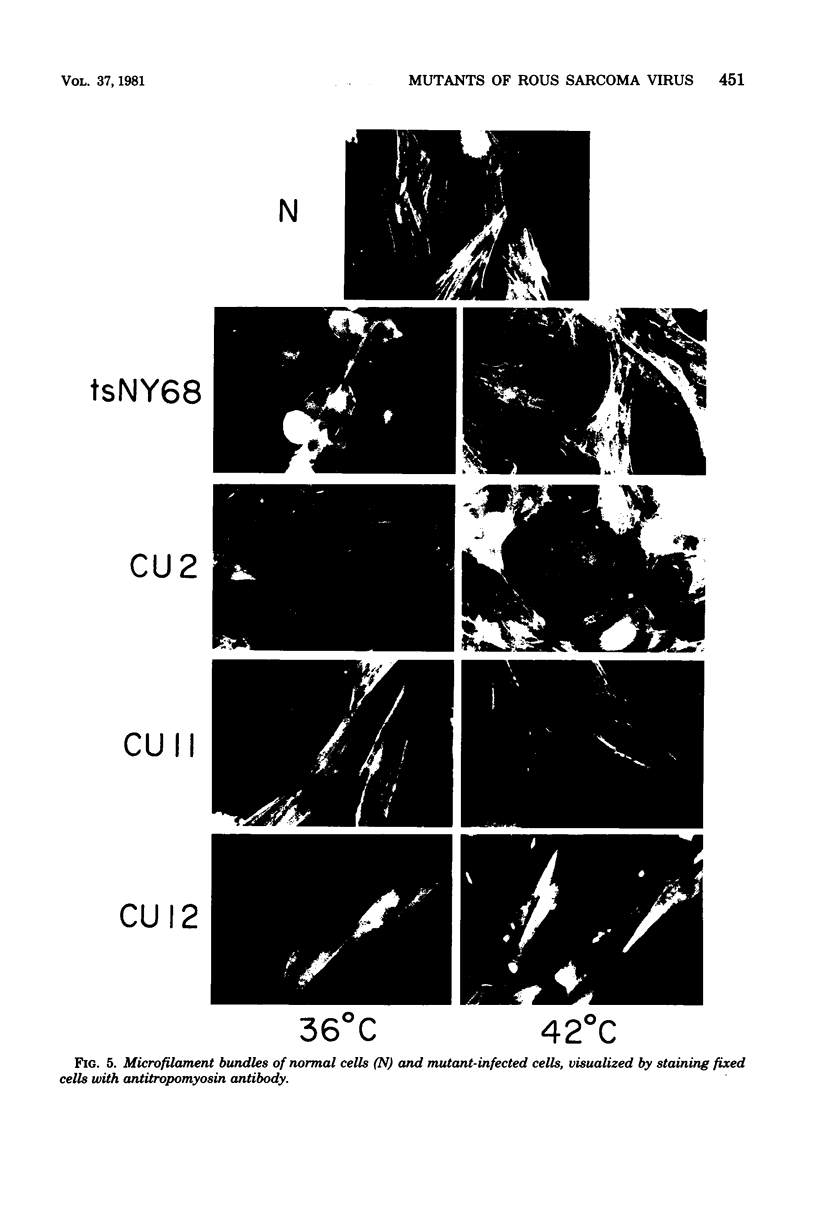
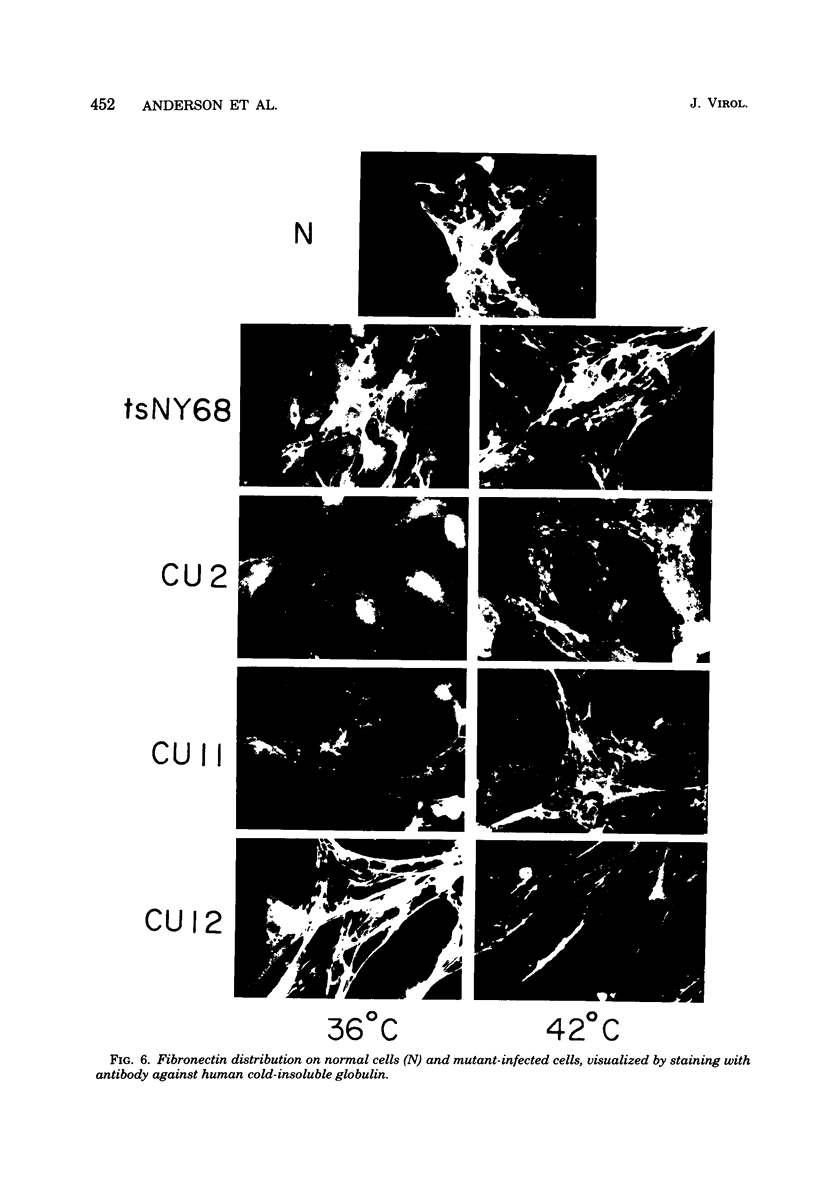
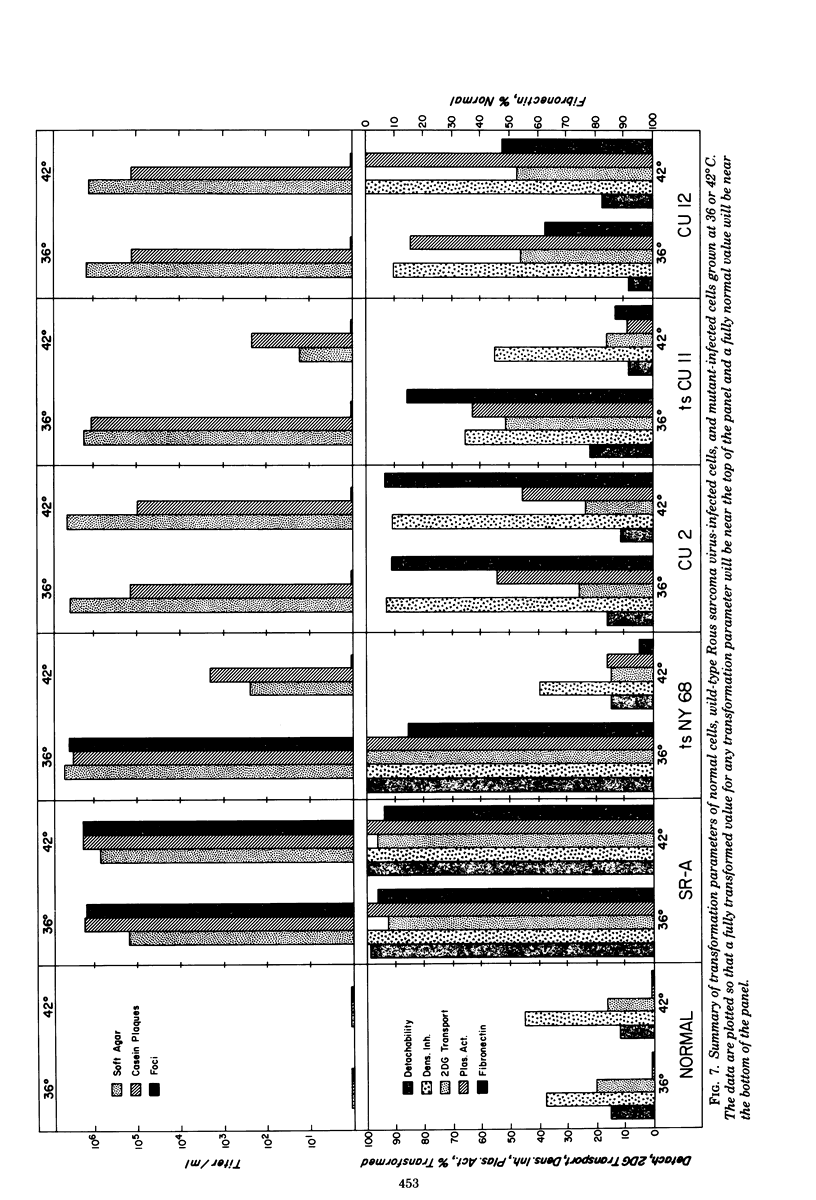
We have isolated mutants of Rous sarcoma virus from an unmutagenized stock of the Schmidt-Ruppin strain of Rous sarcoma virus. These mutants induce only a "partial" transformation, and the transformation properties induced show unusual properties or combinations. Cells infected with mutant CU2 have a unique "blebby" morphology, have lost surface fibronectin, form very small colonies in soft agar, and are nearly normal with respect to adhesiveness and hexose transport. Cells infected with mutant tsCU11 have a nearly normal morphology, but grow well in soft agar. Cells infected with mutant CU12 have a fusiform morphology, intermediate levels of hexose transport and fibronectin, and form very large colonies in soft agar. Because the appearance of the different parameters of transformation is dissociated in these mutant-infected cells, these data are interpreted as supporting a model in which the transforming protein pp60src interacts with more than one primary target in generating the transformed phenotype. All of the mutants display levels of pp60src kinase activity less than that of the wild type. In the case of mutant CU12, the lower kinase activity is in part a consequence of a lower steady-state amount of pp60src inside the cell.
Full text
PDF

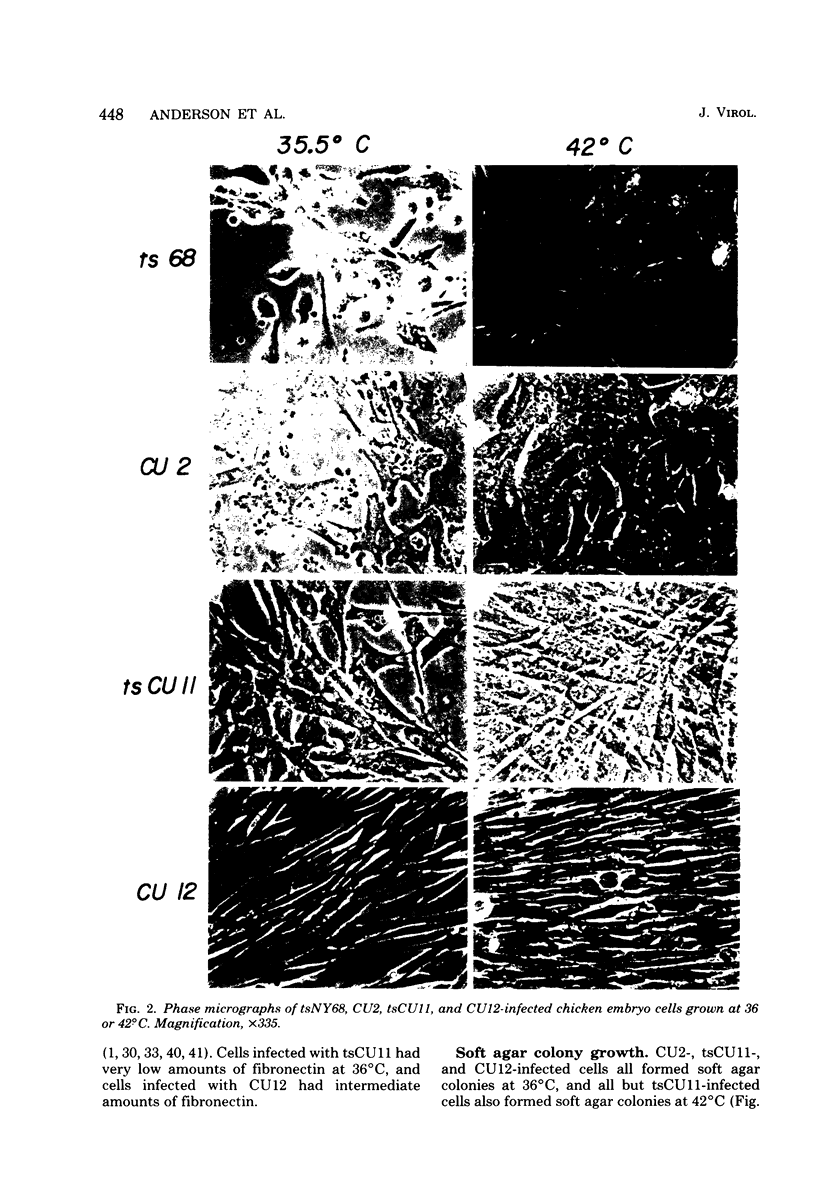
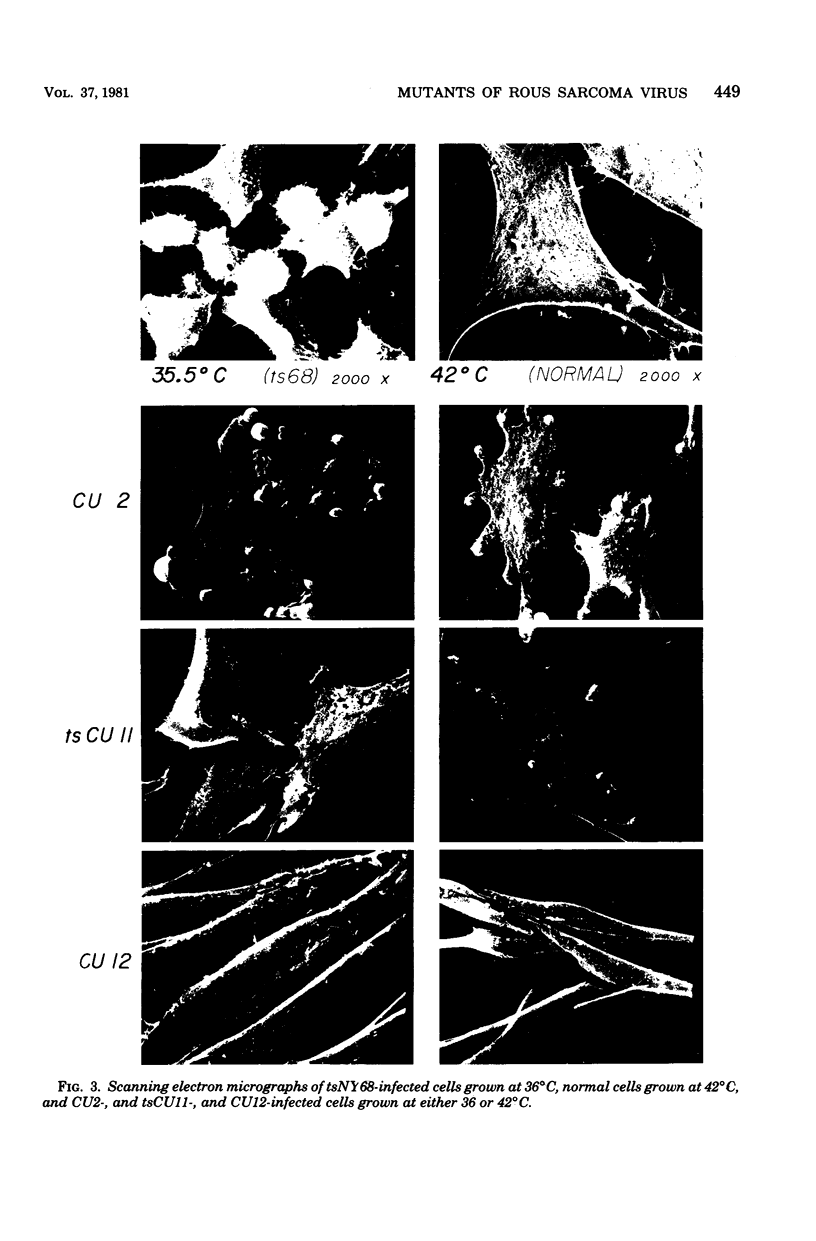




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Mautner V., Lanza R., Hynes R. O. Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell. 1977 May;11(1):115–126. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- Balduzzi P. C., Murphy H. Plaque assay of avian sarcoma viruses using casein. J Virol. 1975 Sep;16(3):707–711. doi: 10.1128/jvi.16.3.707-711.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D., Kurth R., Critchley D., Friis R., Bauer H. Distinguishable transformation-defective phenotypes among temperature-sensitive mutants of Rous sarcoma virus. J Virol. 1977 Mar;21(3):1042–1055. doi: 10.1128/jvi.21.3.1042-1055.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Claviez M., Jockusch B. M., Graf T. Differential expression of Rous Sarcoma virus-specific transformation parameters in enucleated cells. Cell. 1978 Aug;14(4):843–856. doi: 10.1016/0092-8674(78)90340-9. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Burridge K., Feramisco J. R. Microinjection and localization of a 130K protein in living fibroblasts: a relationship to actin and fibronectin. Cell. 1980 Mar;19(3):587–595. doi: 10.1016/s0092-8674(80)80035-3. [DOI] [PubMed] [Google Scholar]
- Calothy G., Pessac B. Growth stimulation of chicl embryo neuroretinal cells infected with Rous sarcoma virus: relationship to viral replication and morphological transformation. Virology. 1976 May;71(1):336–345. doi: 10.1016/0042-6822(76)90117-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness M. H., Ash J. F., Singer S. J. Transmembrane linkage of fibronectin to intracellular actin-containing filaments in cultured human fibroblasts. Ann N Y Acad Sci. 1978 Jun 20;312:414–417. doi: 10.1111/j.1749-6632.1978.tb16822.x. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. Relationships between fibronectin (LETS protein) and actin. Cell. 1978 Nov;15(3):875–886. doi: 10.1016/0092-8674(78)90272-6. [DOI] [PubMed] [Google Scholar]
- Kawai S., Duesberg P. H., Hanafusa H. Transformation-defective mutants of Rous sarcoma virus with src gene deletions of varying length. J Virol. 1977 Dec;24(3):910–914. doi: 10.1128/jvi.24.3.910-914.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau A. F., Krzyzek R. A., Brugge J. S., Erikson R. L., Schollmeyer J., Faras A. J. Morphological revertants of an avian sarcoma virus-transformed mammalian cell line exhibit tumorigenicity and contain pp60src. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3904–3908. doi: 10.1073/pnas.76.8.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Franchi A., Salomon J. C., Silvestre P. Isolation of a Chinese hamster fibroblast mutant defective in hexose transport and aerobic glycolysis: its use to dissect the malignant phenotype. Proc Natl Acad Sci U S A. 1980 May;77(5):2698–2701. doi: 10.1073/pnas.77.5.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke K., Martin G. S. Transformation by Rous sarcoma virus: effects of src gene expression on the synthesis and phosphorylation of cellular polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5212–5216. doi: 10.1073/pnas.76.10.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R. Adhesion plaques of Rous sarcoma virus-transformed cells contain the src gene product. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3514–3518. doi: 10.1073/pnas.77.6.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R. Immunofluorescence on avian sarcoma virus-transformed cells: localization of the src gene product. Cell. 1979 Jan;16(1):11–24. doi: 10.1016/0092-8674(79)90183-1. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Beug H., Claviez M., Winkhardt H. J., Friis R. R., Graf T. Transformation parameters in chicken fibroblasts transformed by AEV and MC29 avian leukemia viruses. Cell. 1978 Apr;13(4):751–760. doi: 10.1016/0092-8674(78)90225-8. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K. Temperature-sensitive transformation by Rous sarcoma virus and temperature-sensitive protein kinase activity. J Virol. 1980 Jan;33(1):220–229. doi: 10.1128/jvi.33.1.220-229.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I. The fibronexus: a transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell. 1979 Mar;16(3):675–685. doi: 10.1016/0092-8674(79)90040-0. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. Separation of morphological conversion and virus production in Rous sarcoma virus infection. Cold Spring Harb Symp Quant Biol. 1962;27:407–414. doi: 10.1101/sqb.1962.027.001.038. [DOI] [PubMed] [Google Scholar]
- Wang E., Goldberg A. R. Changes in microfilament organization and surface topogrophy upon transformation of chick embryo fibroblasts with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4065–4069. doi: 10.1073/pnas.73.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Bibring T., Osborn M. Specific visualization of tubulin-containing structures in tissue culture cells by immunofluorescence. Cytoplasmic microtubules, vinblastine-induced paracrystals, and mitotic figures. Exp Cell Res. 1975 Oct 1;95(1):111–120. doi: 10.1016/0014-4827(75)90615-1. [DOI] [PubMed] [Google Scholar]
- Weber M. J., Friis R. R. Dissociation of transformation parameters using temperature-conditional mutants of Rous sarcoma virus. Cell. 1979 Jan;16(1):25–32. doi: 10.1016/0092-8674(79)90184-3. [DOI] [PubMed] [Google Scholar]
- Weber M. J., Hale A. H., Losasso L. Decreased adherence to the substrate in Rous sarcoma virus-transformed chicken embryo fibroblasts. Cell. 1977 Jan;10(1):45–51. doi: 10.1016/0092-8674(77)90138-6. [DOI] [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]