Abstract
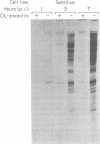
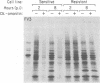
The involvement of host cell RNA polymerase II in the replication of frog virus 3 (FV 3) was examined in alpha-amanitin-sensitive or -resistant Chinese hamster ovary (CHO) cells in the presence and absence of alpha-amanitin. In the presence of alpha-amanitin, FV 3 replicated normally in resistant CHO cells but failed to do so in sensitive CHO cells. Synthesis of virus-specific RNAs and proteins was inhibited in sensitive cells infected in the presence of alpha-amanitin, but in alpha-amanitin-resistant cells, as expected, virus-specific protein synthesis and, by implication, virus-specific RNA synthesis were not affected by the presence of the drug. Inhibition of FV 3 replication was maximum when alpha-amanitin was added to sensitive CHO cells before virus adsorption, but the drug had no effect on virus replication if added after the adsorption. These data indicate that host RNA polymerase II was required for early transcription of the FV 3 genome and confirm a nuclear requirement for FV 3 RNA synthesis (R. Goorha et al., Virology 82:34-52, 1978).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amati P., Blasi F., Di Porzio U., Riccio A., Traboni C. Hamster alpha-amanitine-resistant RNA polymerase II able to transcribe polyoma virus genome in somatic cell hybrids. Proc Natl Acad Sci U S A. 1975 Feb;72(2):753–757. doi: 10.1073/pnas.72.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubertin A. M., Travo C., Kirn A. Proteins solubilized from frog virus 3 particles: effect on transcription. J Virol. 1976 Apr;18(1):34–41. doi: 10.1128/jvi.18.1.34-41.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Costanzo F., Foa'-Tomasi L., La Placa M. Modifications of cellular RNA-polymerase II after infection with frog virus 3. J Gen Virol. 1975 Jun;27(3):391–394. doi: 10.1099/0022-1317-27-3-391. [DOI] [PubMed] [Google Scholar]
- Chan V. L., Whitmore G. F., Siminovitch L. Mammalian cells with altered forms of RNA polymerase II. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3119–3123. doi: 10.1073/pnas.69.11.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaby N. S., Kucera L. S. DNA-dependent RNA polymerase activity associated with subviral particles of polyhedral cytoplasmic dexoyribovirus. J Virol. 1974 Aug;14(2):231–238. doi: 10.1128/jvi.14.2.231-238.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorha R., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. I. Virus-specific protein synthesis and its regulation. Virology. 1974 Jul;60(1):237–250. doi: 10.1016/0042-6822(74)90381-x. [DOI] [PubMed] [Google Scholar]
- Goorha R., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. II. Evidence for post-transcriptional control of a viral structural protein. Virology. 1974 Jul;60(1):251–259. doi: 10.1016/0042-6822(74)90382-1. [DOI] [PubMed] [Google Scholar]
- Goorha R., Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. VI. Frog virus 3 replication is dependent on the cell nucleus. J Virol. 1977 Feb;21(2):802–805. doi: 10.1128/jvi.21.2.802-805.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. C., Robertson J. S. Icosahedral cytoplasmic deoxyriboviruses. J Gen Virol. 1973 Jun;20(Suppl):17–41. doi: 10.1099/0022-1317-20-Supplement-17. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Synthesis of influenza virus polypeptides in cells resistant to alpha-amanitin: evidence for the involvement of cellular RNA polymerase II in virus replication. J Virol. 1977 Sep;23(3):816–819. doi: 10.1128/jvi.23.3.816-819.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naegele R. F., Granoff A. Viruses and renal carcinoma of Rana pipiens. XI. Isolation of frog virus 3 temperature-sensitive mutants; complementation and genetic recombination. Virology. 1971 May;44(2):286–295. doi: 10.1016/0042-6822(71)90260-1. [DOI] [PubMed] [Google Scholar]
- Raghow R., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. XIV. Characterization of the methylated nucleotide sequences in viral messenger RNAs. Virology. 1980 Nov;107(1):283–294. doi: 10.1016/0042-6822(80)90293-7. [DOI] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Specific nucleolar and nucleoplasmic RNA polymerases. Proc Natl Acad Sci U S A. 1970 Mar;65(3):675–682. doi: 10.1073/pnas.65.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers D. G., Pearson M. L., Ingles C. J. Isolation and characterization of an alpha-amanitin-resistant rat myoblast mutant cell line possessing alpha-amanitin-resistant RNA polymerase II. J Biol Chem. 1975 Jul 10;250(13):4825–4831. [PubMed] [Google Scholar]
- Willis D. B., Goorha R., Miles M., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. VII. Transcriptional and post-transcriptional regulation of virus gene expression. J Virol. 1977 Oct;24(1):326–342. doi: 10.1128/jvi.24.1.326-342.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]