Abstract
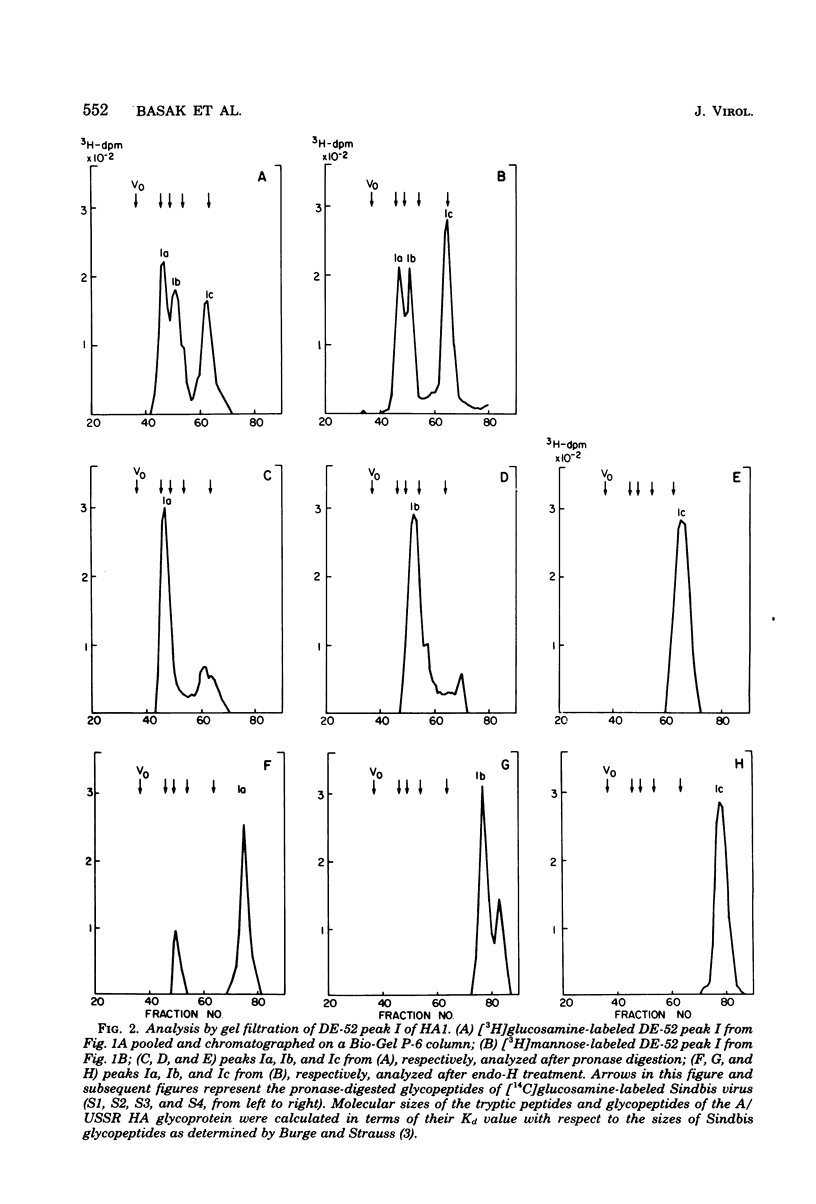
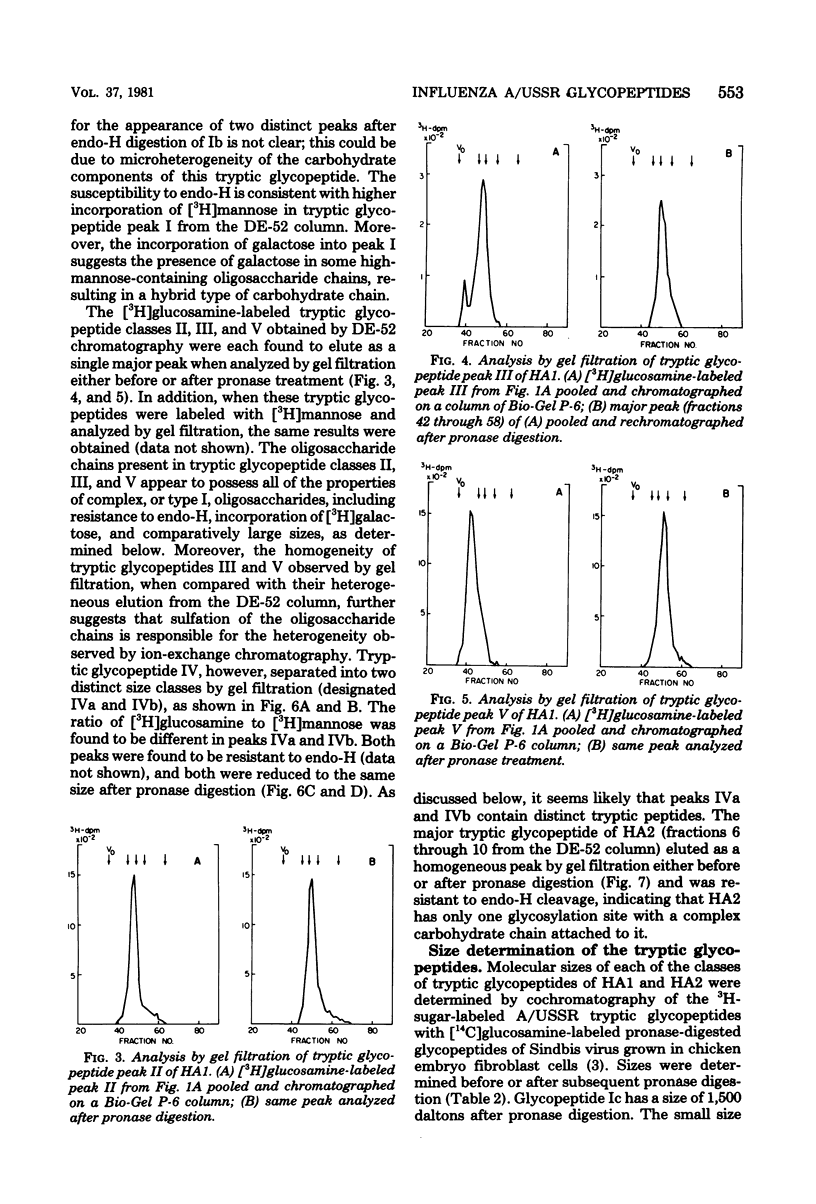
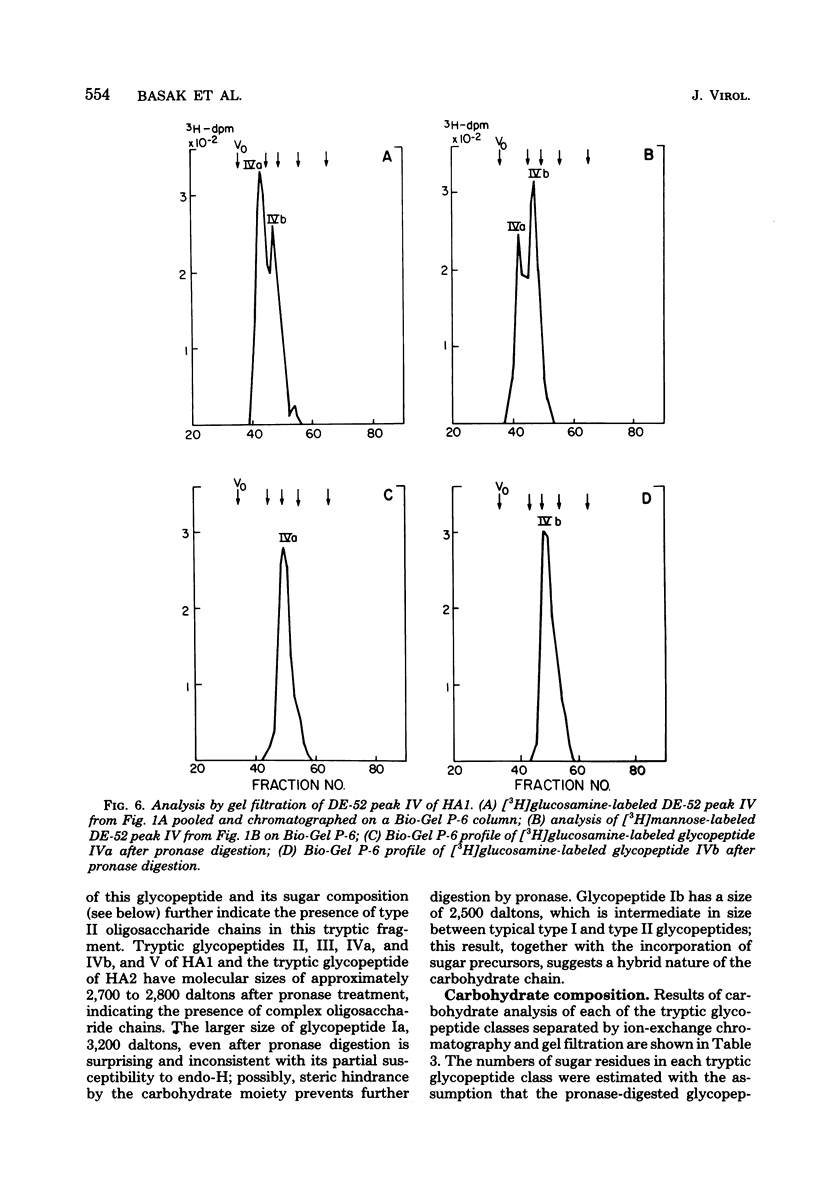
Glycosylated tryptic peptides of the hemagglutinin (HA) glycoprotein of influenza A/USSR/90/77(H1N1) virus were separated by a combination of ion-exchange chromatography and gel filtration. Seven different glycosylated tryptic peptide classes were obtained from the HA1 polypeptide, and only one glycosylated peptide was obtained from the HA2 polypeptide. Several of the tryptic fragments of HA1 and the HA2 glycopeptides were sulfated. The nature of the carbohydrate chain in each of the glycosylated tryptic peptides was determined from observations of the incorporation of different sugar precursors and susceptibility to cleavage by the enzyme endoglycosidase H and by compositional analysis by gas chromatography. Such analyses showed that three types of carbohydrate chains were present in HA1 (type I [complex], type II [high mannose], and hybrid type), whereas HA2 contained only type I oligosaccharide chains. The amino acid composition of each of the glycosylated tryptic peptides was also determined.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa M., Muramatsu T. Endo-beta-N-acetylglucosaminidases acting on the carbohydrate moieties of glycoproteins. The differential specificities of the enzymes from Streptomyces griseus and Diplococcus pneumoniae. J Biochem. 1974 Aug;76(2):307–317. doi: 10.1093/oxfordjournals.jbchem.a130572. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Strauss J. H., Jr Glycopeptides of the membrane glycoprotein of Sindbis virus. J Mol Biol. 1970 Feb 14;47(3):449–466. doi: 10.1016/0022-2836(70)90314-1. [DOI] [PubMed] [Google Scholar]
- Collins J. K., Knight C. A. Purification of the influenza hemagglutinin glycoprotein and characterization of its carbohydrate components. J Virol. 1978 May;26(2):457–467. doi: 10.1128/jvi.26.2.457-467.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Pinter A. Incorporation of sulfate into influenza virus glycoproteins. Virology. 1975 Jul;66(1):151–160. doi: 10.1016/0042-6822(75)90186-5. [DOI] [PubMed] [Google Scholar]
- Elder K. T., Bye J. M., Skehel J. J., Waterfield M. D., Smith A. E. In vitro synthesis, glycosylation, and membrane insertion of influenza virus haemagglutinin. Virology. 1979 Jun;95(2):343–350. doi: 10.1016/0042-6822(79)90489-6. [DOI] [PubMed] [Google Scholar]
- Jackson D. C., Dopheide T. A., Russell R. J., White D. O., Ward C. W. Antigenic determinants of influenza virus hemagglutinin. II. Antigenic reactivity of the isolated N-terminal cyanogen bromide peptide of A/Memphis/72 hemagglutinin heavy chain. Virology. 1979 Mar;93(2):458–465. doi: 10.1016/0042-6822(79)90249-6. [DOI] [PubMed] [Google Scholar]
- Jou W. M., Verhoeyen M., Devos R., Saman E., Fang R., Huylebroeck D., Fiers W., Threlfall G., Barber C., Carey N. Complete structure of the hemagglutinin gene from the human influenza A/Victoria/3/75 (H3N2) strain as determined from cloned DNA. Cell. 1980 Mar;19(3):683–696. doi: 10.1016/s0092-8674(80)80045-6. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Kilbourne E. D. Identification in a recombinant influenza virus of structural proteins derived from both parents. Virology. 1966 Nov;30(3):493–501. doi: 10.1016/0042-6822(66)90125-5. [DOI] [PubMed] [Google Scholar]
- Laver W. G. Separation of two polypeptide chains from the hemagglutinin subunit of influenza virus. Virology. 1971 Jul;45(1):275–288. doi: 10.1016/0042-6822(71)90134-6. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Choppin P. W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975 Dec;68(2):440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Leavitt J. C., Phelan M. A., Leavitt A. H., Mayner R. E., Ennis F. A. Human influenza A virus: comparative analysis of the structural polypeptides by two-dimensional polyacrylamide gel electrophoresis. Virology. 1979 Dec;99(2):340–348. doi: 10.1016/0042-6822(79)90013-8. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Bhown A. S., Compans R. W. Glycosylation sites of influenza viral glycoproteins. Tryptic glycopeptides from the A/WSN (H0N1) hemagglutinin glycoprotein. Virology. 1980 Nov;107(1):208–221. doi: 10.1016/0042-6822(80)90286-x. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Glycopeptide components of influenza viral glycoproteins. Virology. 1978 May 15;86(2):432–442. doi: 10.1016/0042-6822(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Host cell- and virus strain-dependent differences in oligosaccharides of hemagglutinin glycoproteins of influenza A viruses. Virology. 1979 May;95(1):8–23. doi: 10.1016/0042-6822(79)90397-0. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. The cellular site of sulfation of influenza viral glycoproteins. Virology. 1977 Jun 15;79(2):381–392. doi: 10.1016/0042-6822(77)90365-8. [DOI] [PubMed] [Google Scholar]
- Nakamura N., Compans R. W. Biosynthesis of the oligosaccharides of influenza viral glycoproteins. Virology. 1979 Feb;93(1):31–47. doi: 10.1016/0042-6822(79)90273-3. [DOI] [PubMed] [Google Scholar]
- Porter A. G., Barber C., Carey N. H., Hallewell R. A., Threlfall G., Emtage J. S. Complete nucleotide sequence of an influenza virus haemagglutinin gene from cloned DNA. Nature. 1979 Nov 29;282(5738):471–477. doi: 10.1038/282471a0. [DOI] [PubMed] [Google Scholar]
- Prehm P., Scheid A., Choppin P. W. The carbohydrate structure of the glycoproteins of the paramyxovirus SV5 grown in bovine kidney cells. J Biol Chem. 1979 Oct 10;254(19):9669–9677. [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- SPIRO R. G. THE CARBOHYDRATE UNITS OF THYROGLOBULIN. J Biol Chem. 1965 Apr;240:1603–1610. [PubMed] [Google Scholar]
- Schwarz R. T., Schmidt M. F., Anwer U., Klenk H. D. Carbohydrates of influenza virus. I. Glycopeptides derived from viral glycoproteins after labeling with radioactive sugars. J Virol. 1977 Aug;23(2):217–226. doi: 10.1128/jvi.23.2.217-226.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverton E. W., Navia M. A., Davies D. R. Three-dimensional structure of an intact human immunoglobulin. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5140–5144. doi: 10.1073/pnas.74.11.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Schild G. C. The polypeptide composition of influenza A viruses. Virology. 1971 May;44(2):396–408. doi: 10.1016/0042-6822(71)90270-4. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Waterfield M. D. Studies on the primary structure of the influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):93–97. doi: 10.1073/pnas.72.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Tokunaga F., Ebrey T. The blue membrane: the 3-dehydroretinal-based artificial pigment of the purple membrane. Biochemistry. 1978 May 16;17(10):1915–1922. doi: 10.1021/bi00603a018. [DOI] [PubMed] [Google Scholar]
- Ward C. W., Dopheide A. A. Size and chemical composition of influenza virus hemagglutinin chains. FEBS Lett. 1976 Jun 15;65(3):365–368. doi: 10.1016/0014-5793(76)80148-2. [DOI] [PubMed] [Google Scholar]
- Ward C. W., Dopheide T. A. Primary structure of the Hong Kong (H3) haemagglutinin. Br Med Bull. 1979 Jan;35(1):51–56. doi: 10.1093/oxfordjournals.bmb.a071542. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Espelie K., Elder K., Skehel J. J. Structure of the haemagglutinin of influenza virus. Br Med Bull. 1979 Jan;35(1):57–63. doi: 10.1093/oxfordjournals.bmb.a071543. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Kobata A. The structures of the galactose-containing sugar chains of ovalbumin. J Biol Chem. 1978 Jun 10;253(11):3862–3869. [PubMed] [Google Scholar]


