Abstract
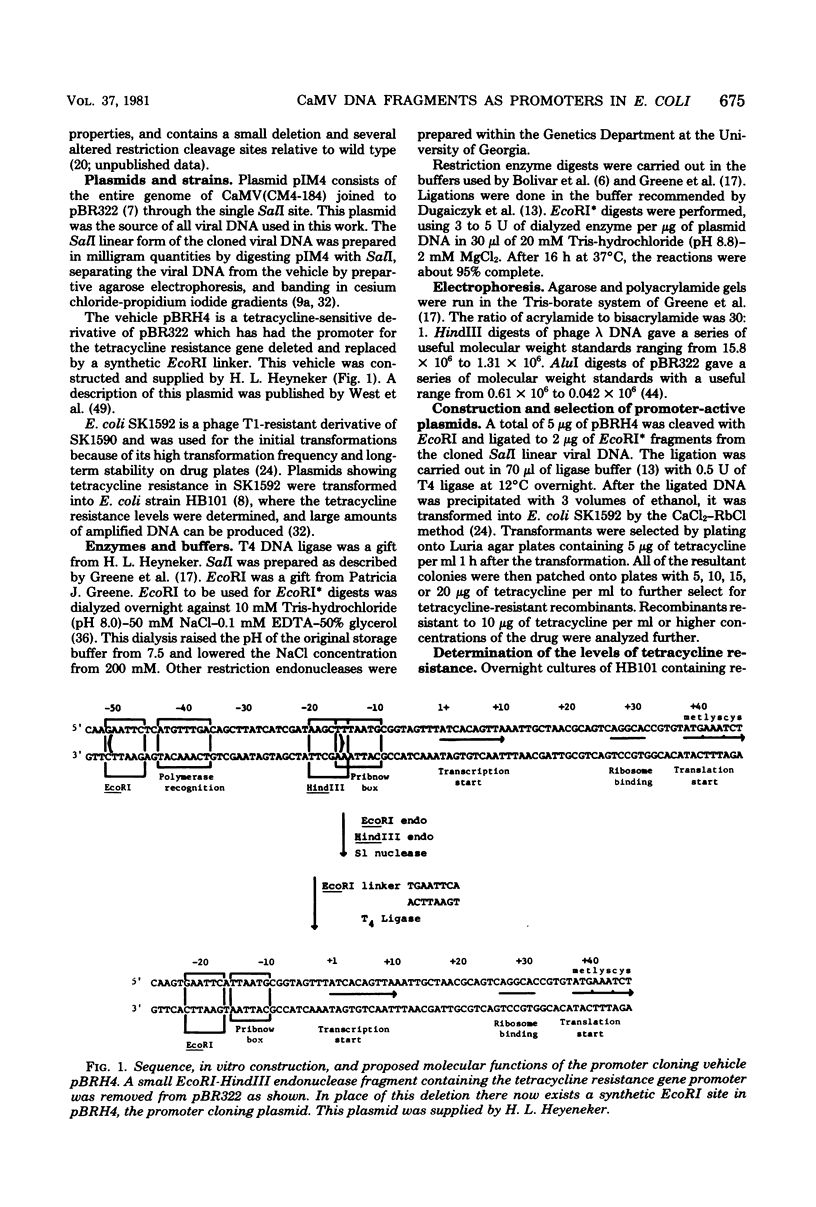
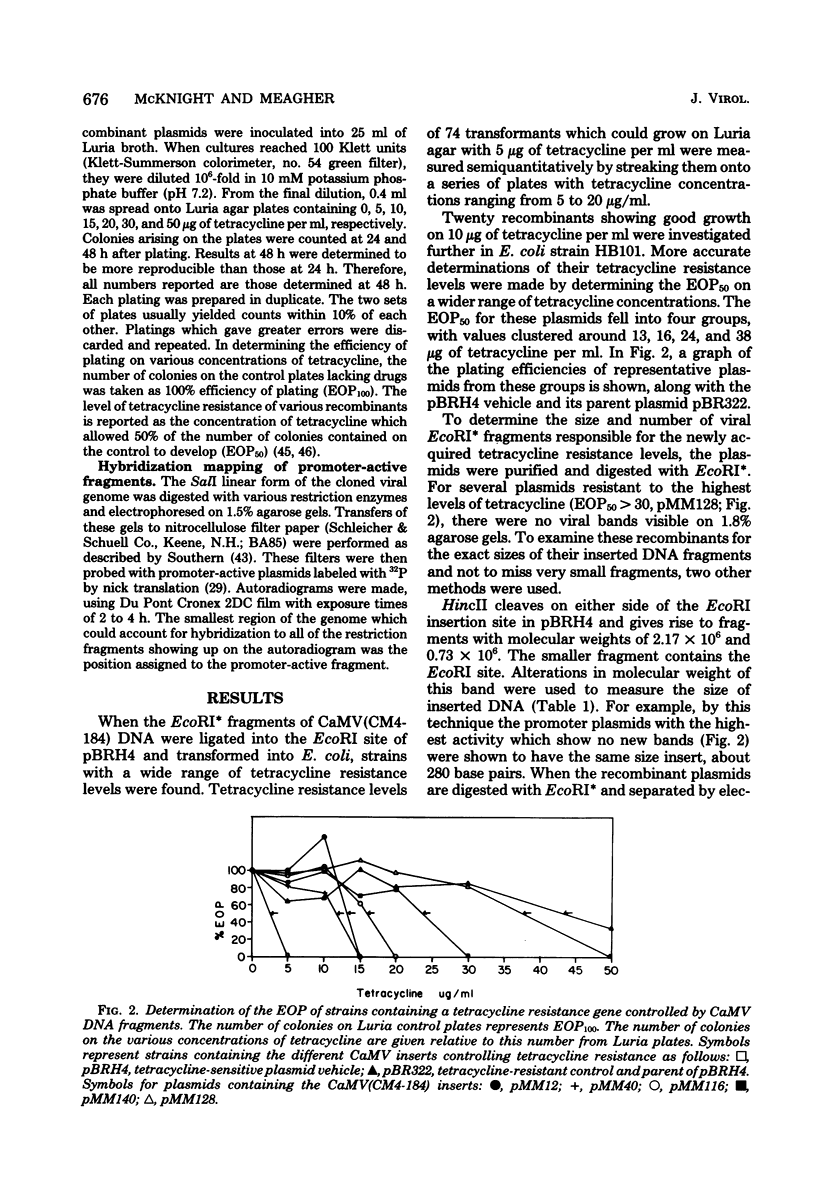
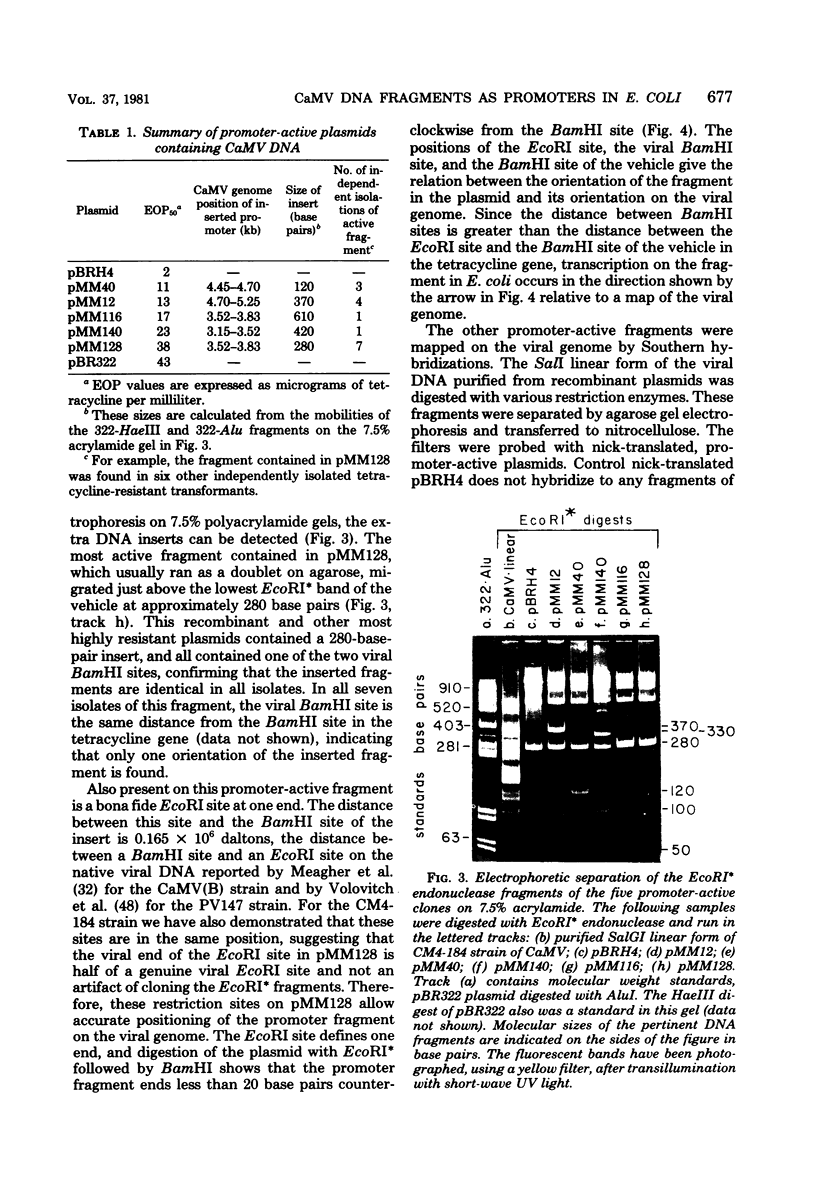
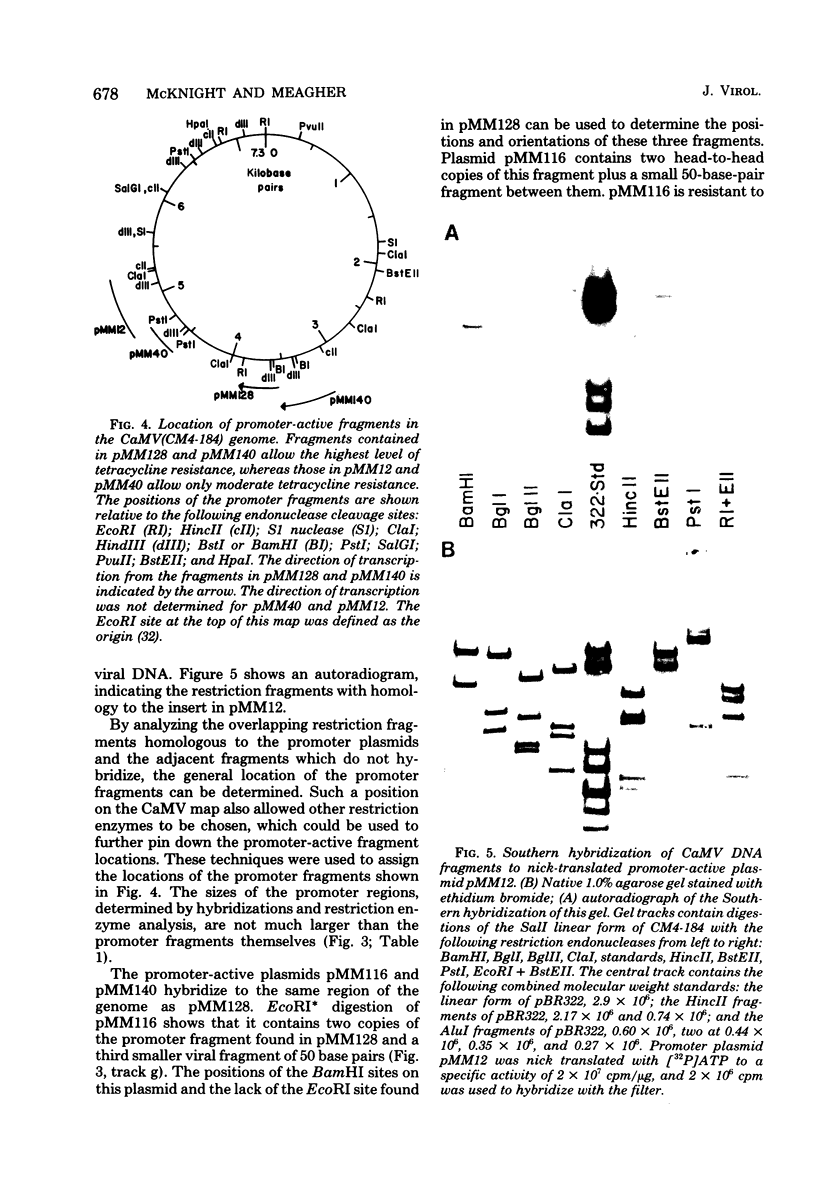
Small EcoRI* fragments of cauliflower mosiac virus DNA (strain CM4-184), which act as promoters for the tetracycline resistance gene on the promoter probe plasmid pBRH4 in Escherichia coli, have been isolated and mapped on the viral genome. Two regions of the viral genome contain DNA sequences with promoter activity in E. coli. Two independent cloned fragments from one region direct a high level of tetracycline resistance (up to 38 μg of tetracycline per ml). Two independent fragments from the second region of the viral genome also direct tetracycline resistance, but at lower levels. The activity of the two fragments with the strongest promoter activity in E. coli may direct transcription of the viral genome in a clockwise direction. This is consistent with the direction of transcription predicted from sequence analysis of the viral DNA (Franck et al., Cell 21: 285-294, 1980). One of these fragments maps at the start of a large open translational reading frame which is predicted to contain the coding sequence for the viral coat protein. Each promoter-active fragment is located in the 5′-terminal portion of one of the six open reading frames predicted from the DNA sequence.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Hautala J. A., Giles N. H., Kushner S. R., Vapnek D. Transcription and translation in E. coli of hybrid plasmids containing the catabolic dehydroquinase gene from Neurospora crassa. Gene. 1978 Nov;4(3):241–259. doi: 10.1016/0378-1119(78)90021-5. [DOI] [PubMed] [Google Scholar]
- Bach M. L., Lacroute F., Botstein D. Evidence for transcriptional regulation of orotidine-5'-phosphate decarboxylase in yeast by hybridization of mRNA to the yeast structural gene cloned in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):386–390. doi: 10.1073/pnas.76.1.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. C., Herisse J., Courtois G., Galibert F., Ziff E. Messenger RNA for the Ad2 DNA binding protein: DNA sequences encoding the first leader and heterogenity at the mRNA 5' end. Cell. 1979 Oct;18(2):569–580. doi: 10.1016/0092-8674(79)90073-4. [DOI] [PubMed] [Google Scholar]
- Battey J., Clayton D. A. The transcription map of mouse mitochondrial DNA. Cell. 1978 May;14(1):143–156. doi: 10.1016/0092-8674(78)90309-4. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Burrell C. J., Mackay P., Greenaway P. J., Hofschneider P. H., Murray K. Expression in Escherichia coli of hepatitis B virus DNA sequences cloned in plasmid pBR322. Nature. 1979 May 3;279(5708):43–47. doi: 10.1038/279043a0. [DOI] [PubMed] [Google Scholar]
- Carreira L. H., Carlton B. C., Bobbio S. M., Nagao R. T., Meagher R. B. Construction and application of a modified "gene machine": a circular concentrating preparative gel electrophoresis device employing discontinuous elution. Anal Biochem. 1980 Aug;106(2):455–468. doi: 10.1016/0003-2697(80)90548-5. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli: specific complementation of argininosuccinate lyase (argH) mutations. J Mol Biol. 1978 Apr 25;120(4):517–532. doi: 10.1016/0022-2836(78)90351-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. D., Eccleshall T. R., Needleman R. B., Federoff H., Buchferer B. A., Marmur J. Functional expression in yeast of the Escherichia coli plasmid gene coding for chloramphenicol acetyltransferase. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1078–1082. doi: 10.1073/pnas.77.2.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Subramanian K. N., Pan J., Weissman S. M. Nucleotide sequence of a fragment of SV40 DNA that contains the origin of DNA replication and specifies the 5' ends of "early" and "late" viral RNA. IV. Localization of the SV40 DNA complementary to the 5' ends of viral mRNA. J Biol Chem. 1977 Jan 10;252(1):368–376. [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Franck A., Guilley H., Jonard G., Richards K., Hirth L. Nucleotide sequence of cauliflower mosaic virus DNA. Cell. 1980 Aug;21(1):285–294. doi: 10.1016/0092-8674(80)90136-1. [DOI] [PubMed] [Google Scholar]
- Gannon F., O'Hare K., Perrin F., LePennec J. P., Benoist C., Cochet M., Breathnach R., Royal A., Garapin A., Cami B. Organisation and sequences at the 5' end of a cloned complete ovalbumin gene. Nature. 1979 Mar 29;278(5703):428–434. doi: 10.1038/278428a0. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Kleid D. G., Bolivar F., Heyneker H. L., Yansura D. G., Crea R., Hirose T., Kraszewski A., Itakura K., Riggs A. D. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):106–110. doi: 10.1073/pnas.76.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale P., Woodward R. S., Lebowitz J. E. coli RNA polymerase promoters on superhelical SV40 DNA are highly selective targets for chemical modification. Nature. 1980 Apr 17;284(5757):640–644. doi: 10.1038/284640a0. [DOI] [PubMed] [Google Scholar]
- Hull R., Covey S. N., Stanley J., Davies J. W. The polarity of the cauliflower mosaic virus genome. Nucleic Acids Res. 1979 Oct 10;7(3):669–677. doi: 10.1093/nar/7.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R., Howell S. H. Structure of the cauliflower mosaic virus genome. II. Variation in DNA structure and sequence between isolates. Virology. 1978 May 15;86(2):482–493. doi: 10.1016/0042-6822(78)90087-9. [DOI] [PubMed] [Google Scholar]
- Itakura K., Hirose T., Crea R., Riggs A. D., Heyneker H. L., Bolivar F., Boyer H. W. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977 Dec 9;198(4321):1056–1063. doi: 10.1126/science.412251. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Kushner S. R. In vivo studies of temperature-sensitive recB and recC mutants. J Bacteriol. 1974 Dec;120(3):1213–1218. doi: 10.1128/jb.120.3.1213-1218.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeurier G., Whitechurch O., Lesot A., Hirth L. Physical map of DNA from a new cauliflower mosaic virus strain. Gene. 1978 Nov;4(3):213–226. doi: 10.1016/0378-1119(78)90019-7. [DOI] [PubMed] [Google Scholar]
- Lescure B., Chestier A., Yaniv M. Transcription of polyoma virus DNA in vitro. II. Transcription of superhelical and linear polyoma DNA by RNA polymerase II. J Mol Biol. 1978 Sep 5;124(1):73–85. doi: 10.1016/0022-2836(78)90148-1. [DOI] [PubMed] [Google Scholar]
- Lescure B., Dauguet C., Yaniv M. Transcription of polyoma virus DNA in vitro. III. Localization of calf thymus RNA polymerase II binding sites. J Mol Biol. 1978 Sep 5;124(1):87–96. doi: 10.1016/0022-2836(78)90149-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher R. B., Shepherd R. J., Boyer H. W. The structure of cauliflower mosaic virus. I. A restriction endonuclease map of cauliflower mosaic virus DNA. Virology. 1977 Jul 15;80(2):362–375. doi: 10.1016/s0042-6822(77)80012-3. [DOI] [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Meagher R. B. The development of a molecular cloning system in higher plants. Basic Life Sci. 1977;9:129–158. doi: 10.1007/978-1-4684-0880-5_11. [DOI] [PubMed] [Google Scholar]
- Nagata S., Taira H., Hall A., Johnsrud L., Streuli M., Ecsödi J., Boll W., Cantell K., Weissmann C. Synthesis in E. coli of a polypeptide with human leukocyte interferon activity. Nature. 1980 Mar 27;284(5754):316–320. doi: 10.1038/284316a0. [DOI] [PubMed] [Google Scholar]
- Neve R. L., West R. W., Rodriguez R. L. Eukaryotic DNA fragments which act as promoters for a plasmid gene. Nature. 1979 Jan 25;277(5694):324–325. doi: 10.1038/277324a0. [DOI] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Ratzkin B., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):487–491. doi: 10.1073/pnas.74.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R. L., West R. W., Heyneker H. L., Bolivar F., Boyer H. W. Characterizing wild-type and mutant promoters of the tetracycline resistance gene in pBR313. Nucleic Acids Res. 1979 Jul 25;6(10):3267–3287. doi: 10.1093/nar/6.10.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Scherer G. E., Walkinshaw M. D., Arnott S. A computer aided oligonucleotide analysis provides a model sequence for RNA polymerase-promoter recognition in E.coli. Nucleic Acids Res. 1978 Oct;5(10):3759–3773. doi: 10.1093/nar/5.10.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. B. The molecular topography of RNA polymerase-promoter interaction. Cell. 1979 Oct;18(2):277–285. doi: 10.1016/0092-8674(79)90047-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait R. C., Boyer H. W. On the nature of tetracycline resistance controlled by the plasmid pSC101. Cell. 1978 Jan;13(1):73–81. doi: 10.1016/0092-8674(78)90139-3. [DOI] [PubMed] [Google Scholar]
- Tait R. C., Rodriguez R. L., Boyer H. W. Altered tetracycline resistance in pSC101 recombinant plasmids. Mol Gen Genet. 1977 Mar 16;151(3):327–331. doi: 10.1007/BF00268797. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Hautala J. A., Jacobson J. W., Giles N. H., Kushner S. R. Expression in Escherichia coli K-12 of the structural gene for catabolic dehydroquinase of Neurospora crassa. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3508–3512. doi: 10.1073/pnas.74.8.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volovitch M., Drugeon G., Dumas J. P., Haenni A. L., Yot P. A restriction map of cauliflower mosaic virus DNA (strain PV 147). Mapping of the cleavage sites of HhaI, SacI, AvaI, PvuII, PstI, XbaI, EcoRI, Bg/II, HincII, HpaII and HindII + III. Eur J Biochem. 1979 Oct;100(1):245–255. doi: 10.1111/j.1432-1033.1979.tb02055.x. [DOI] [PubMed] [Google Scholar]
- West R. W., Jr, Neve R. L., Rodriguez R. L. Construction and characterization of E. coli promoter-probe plasmid vectors. I. Cloning of promoter-containing DNA fragments. Gene. 1979 Nov;7(3-4):271–288. doi: 10.1016/0378-1119(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Steitz J. A. Tandem promoters direct E. coli ribosomal RNA synthesis. Cell. 1979 May;17(1):225–234. doi: 10.1016/0092-8674(79)90310-6. [DOI] [PubMed] [Google Scholar]