Abstract
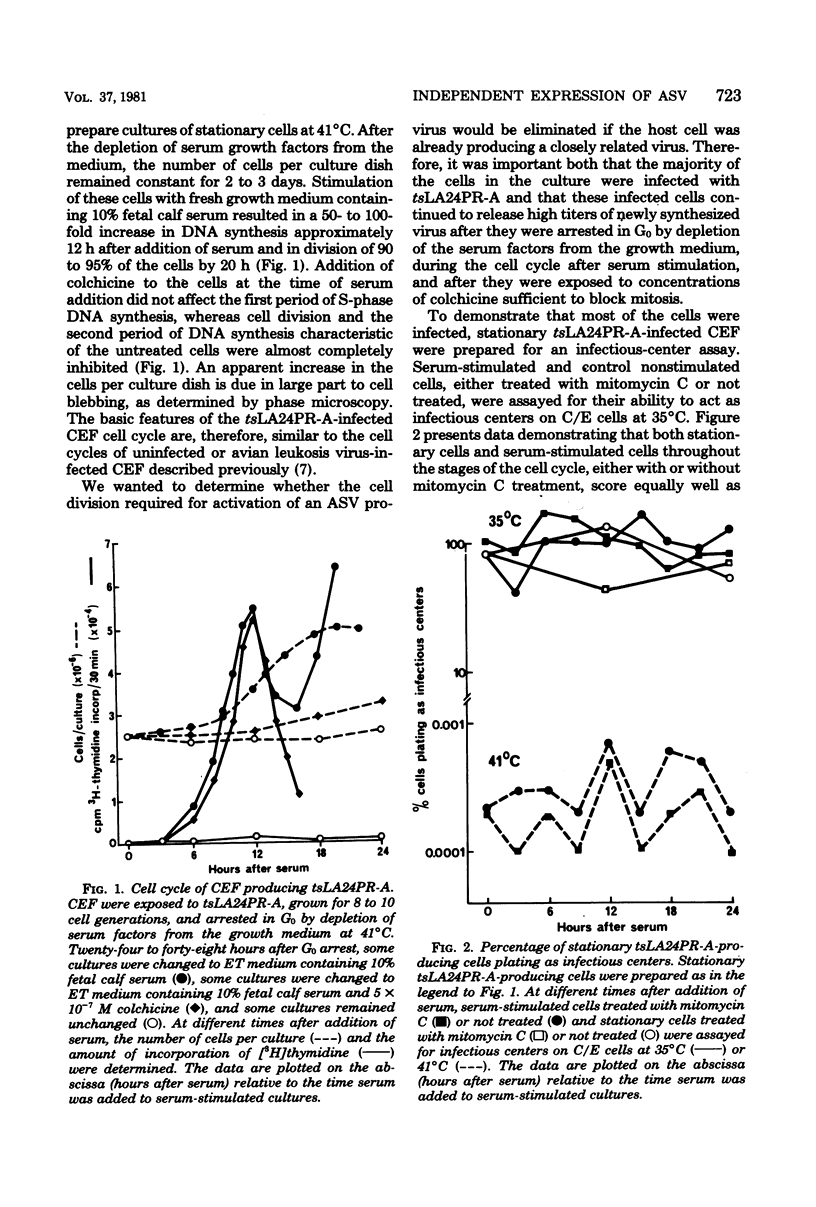
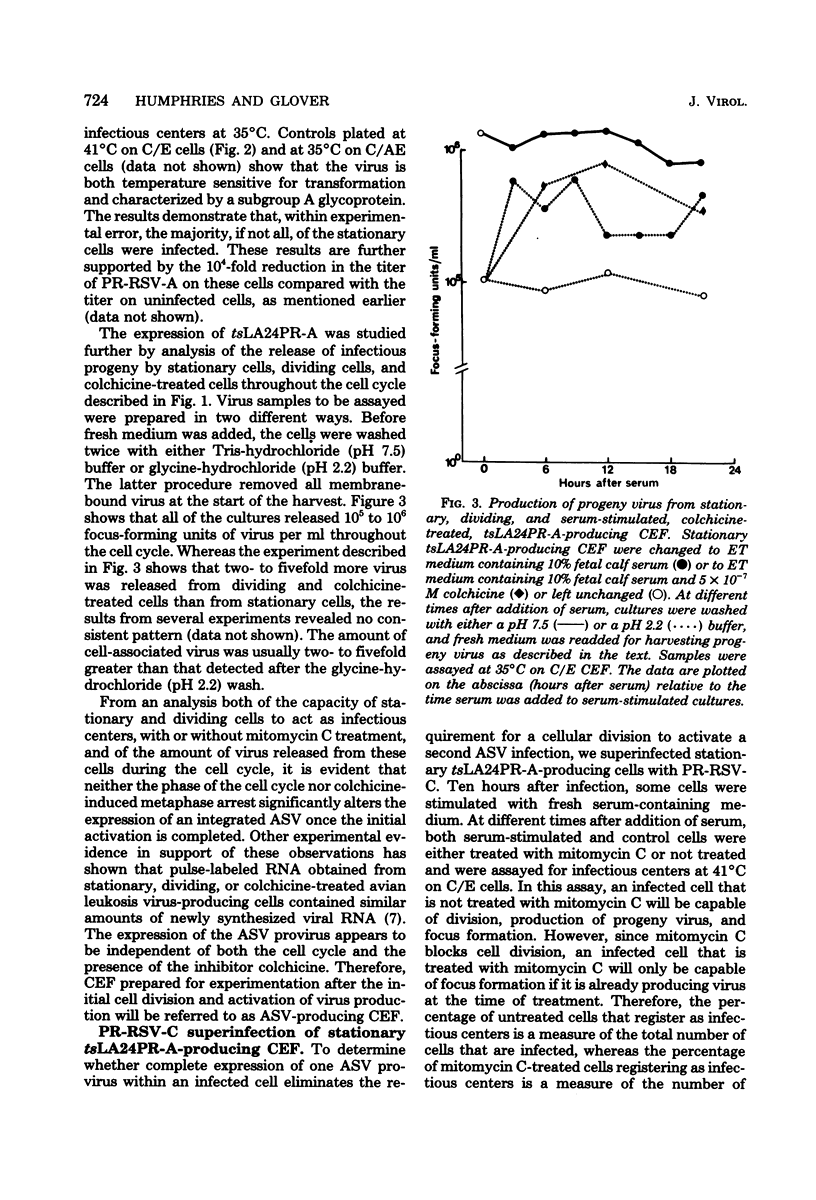
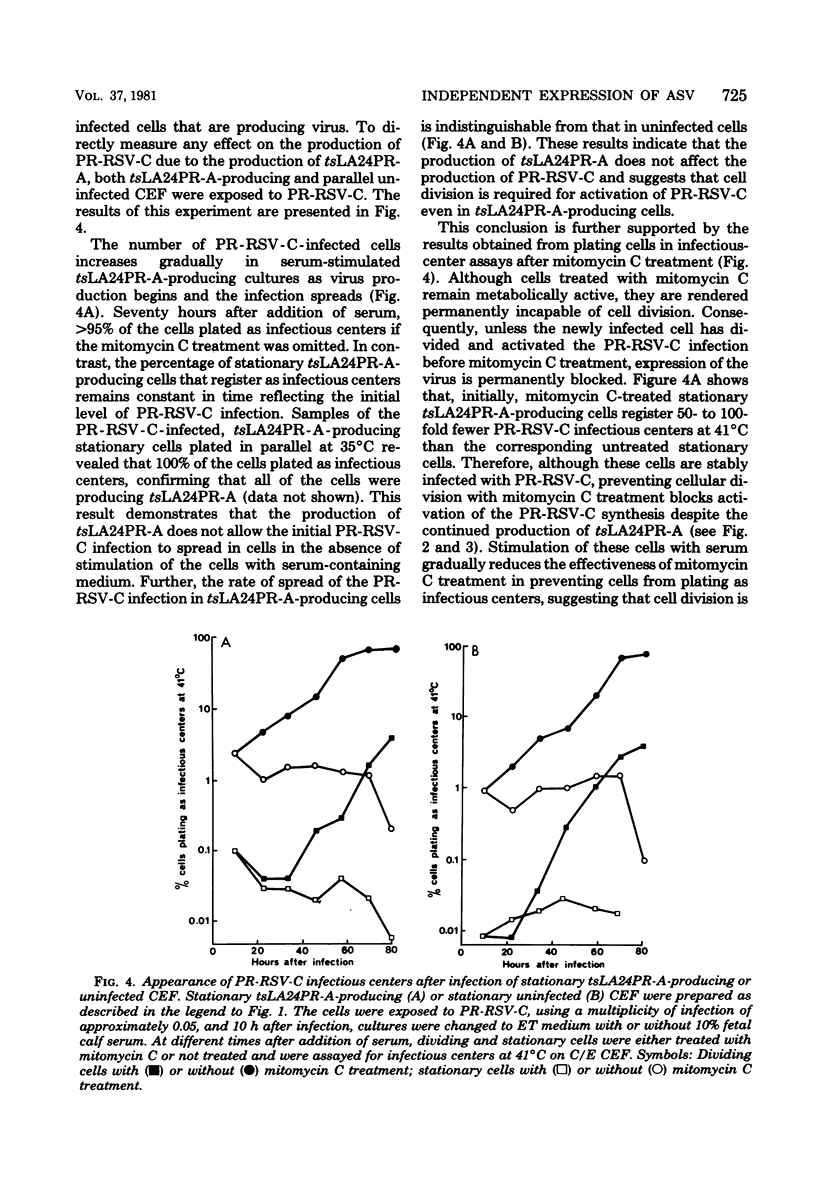
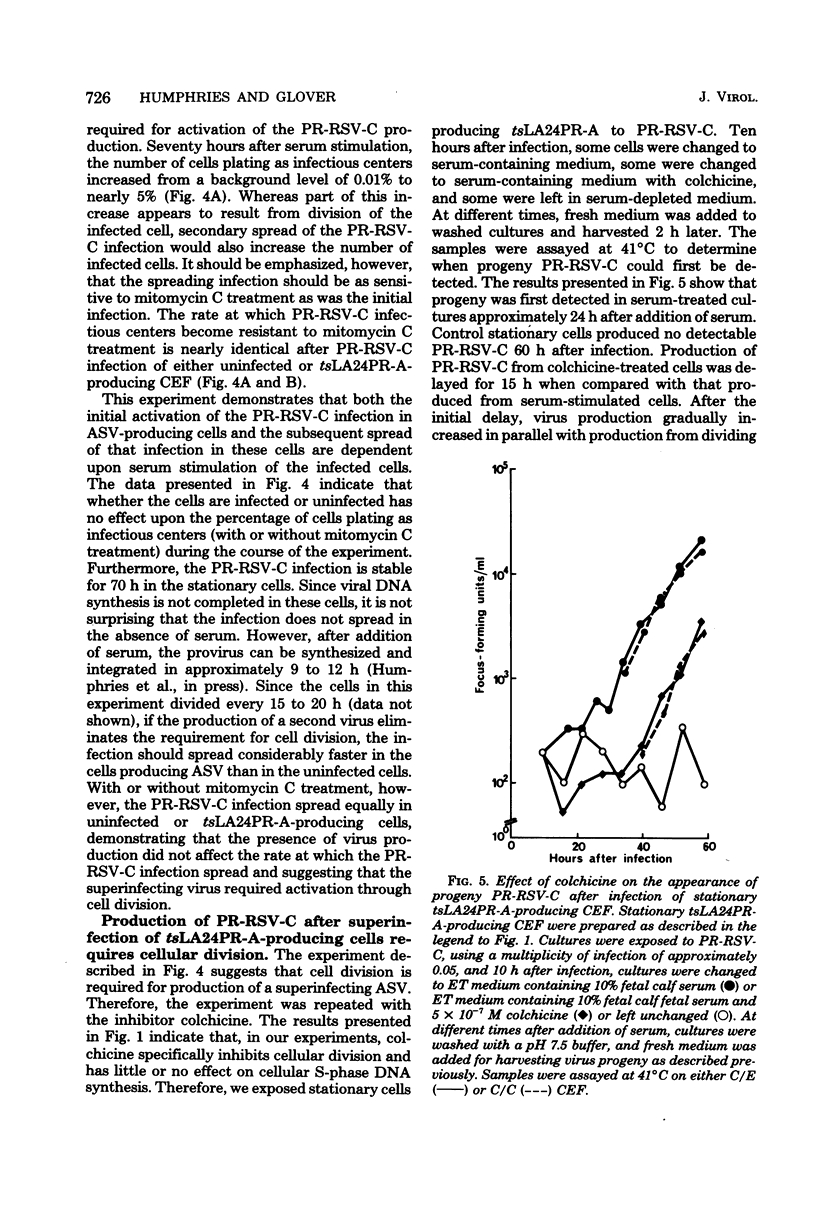
Infection of a chicken cell with avian sarcoma virus requires division of the infected cell before synthesis of infectious progeny is initiated. This requirement for a cell division for the complete expression of avian sarcoma virus has been examined further with chicken embryo fibroblasts infected with two distinct viruses. Chicken cells infected with and producing a mutant of Rous sarcoma virus temperature sensitive for transformation (tsLA24PR-A) were arrested in G0 by depletion of serum factors from growth medium. These stationary cells continued to produce infectious progeny in the absence of further cell division. Superinfection of the stationary cells with the wild-type Prague strain of Rous sarcoma virus (PR-RSV-C) produced a stable double infection in these cells. Progeny of the superinfecting PR-RSV-C, however, were not detected until these cells underwent division after stimulation with fresh serum-containing medium. The addition of colchicine to these serum-stimulated cells, although not affecting production of the tsLA24PR-A, inhibited the appearance of progeny of the superinfecting PR-RSV-C. These experiments indicate that each avian sarcoma virus infection of a chicken embryo fibroblast requires division of the infected cell for production of that virus regardless of whether or not the cell is already producing a similar virus. The results suggest, therefore, that the requirement for a cell division represents a requirement for an event that controls virus expression in a "cis-acting" fashion specific for the provirus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Temin H. M. Light inactivation of focus formation by chicken embryo fibroblasts infected with avian sarcoma virus in the presence of 5-bromodeoxyuridine. Nature. 1970 Nov 14;228(5272):622–624. doi: 10.1038/228622a0. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Taylor E. W. The mechanism of action of colchicine. Colchicine binding to sea urchin eggs and the mitotic apparatus. J Cell Biol. 1967 Aug;34(2):535–548. doi: 10.1083/jcb.34.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ege T., Ringertz N. R. Preparation of microcells by enucleation of micronucleate cells. Exp Cell Res. 1974 Aug;87(2):378–382. doi: 10.1016/0014-4827(74)90494-7. [DOI] [PubMed] [Google Scholar]
- Fritsch E. F., Temin H. M. Inhibition of viral DNA synthesis in stationary chicken embryo fibroblasts infected with avian retroviruses. J Virol. 1977 Nov;24(2):461–469. doi: 10.1128/jvi.24.2.461-469.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries E. H., Coffin J. M. Rate of virus-specific RNA synthesis in synchronized chicken embryo fibroblasts infected with avian leukosis virus. J Virol. 1976 Feb;17(2):393–401. doi: 10.1128/jvi.17.2.393-401.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries E. H., Temin H. M. Cell cycle-dependent activation of rous sarcoma virus-infected stationary chicken cells: avian leukosis virus group-specific antigens and ribonucleic acid. J Virol. 1972 Jul;10(1):82–87. doi: 10.1128/jvi.10.1.82-87.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries E. H., Temin H. M. Requirement for cell division for initiation of transcription of Rous sarcoma virus RNA. J Virol. 1974 Sep;14(3):531–546. doi: 10.1128/jvi.14.3.531-546.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy H. M. A new replication-defective variant of the Bryan high-titer strain Rous sarcoma virus. Virology. 1977 Apr;77(2):705–721. doi: 10.1016/0042-6822(77)90493-7. [DOI] [PubMed] [Google Scholar]
- Steck F. T., Rubin H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. I. Establishment of interference. Virology. 1966 Aug;29(4):628–641. doi: 10.1016/0042-6822(66)90287-x. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Stimulation by serum of multiplication of stationary chicken cells. J Cell Physiol. 1971 Oct;78(2):161–170. doi: 10.1002/jcp.1040780202. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Padgett T., Heasley S., Simon G., Bishop J. M. Cellular functions are required for the synthesis and integration of avian sarcoma virus-specific DNA. Cell. 1977 Jun;11(2):307–319. doi: 10.1016/0092-8674(77)90047-2. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Weiss R. A., Hanafusa H. Proposal for numbering mutants of avian leukosis and sarcoma viruses. J Virol. 1974 Feb;13(2):551–554. doi: 10.1128/jvi.13.2.551-554.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]


