Abstract
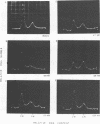
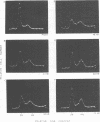
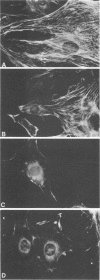
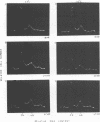
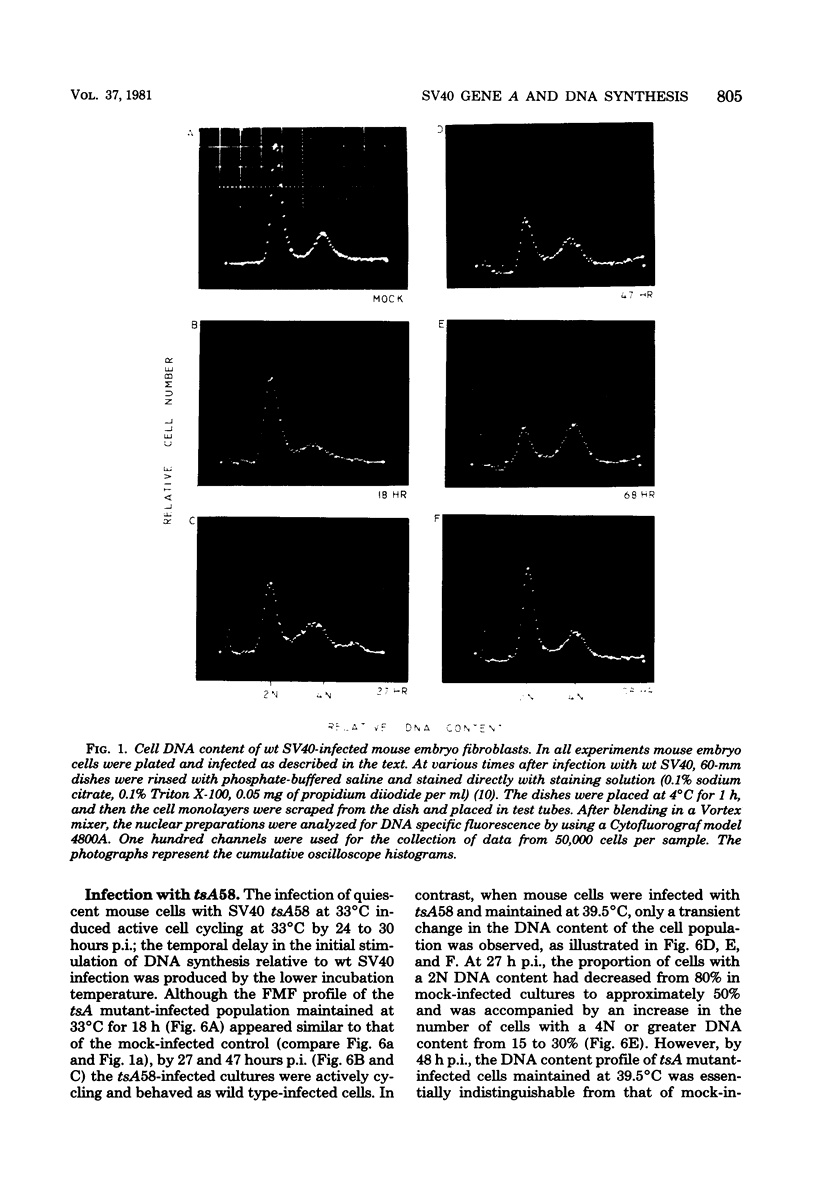
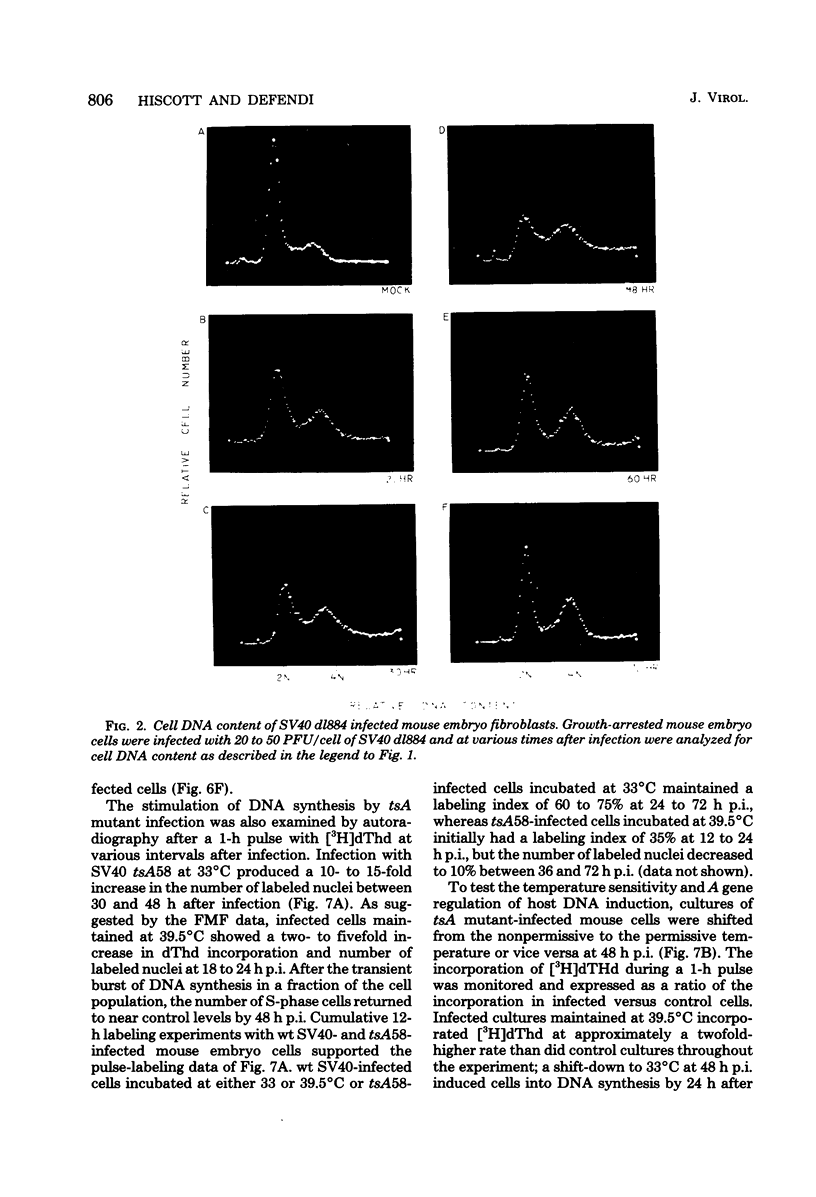
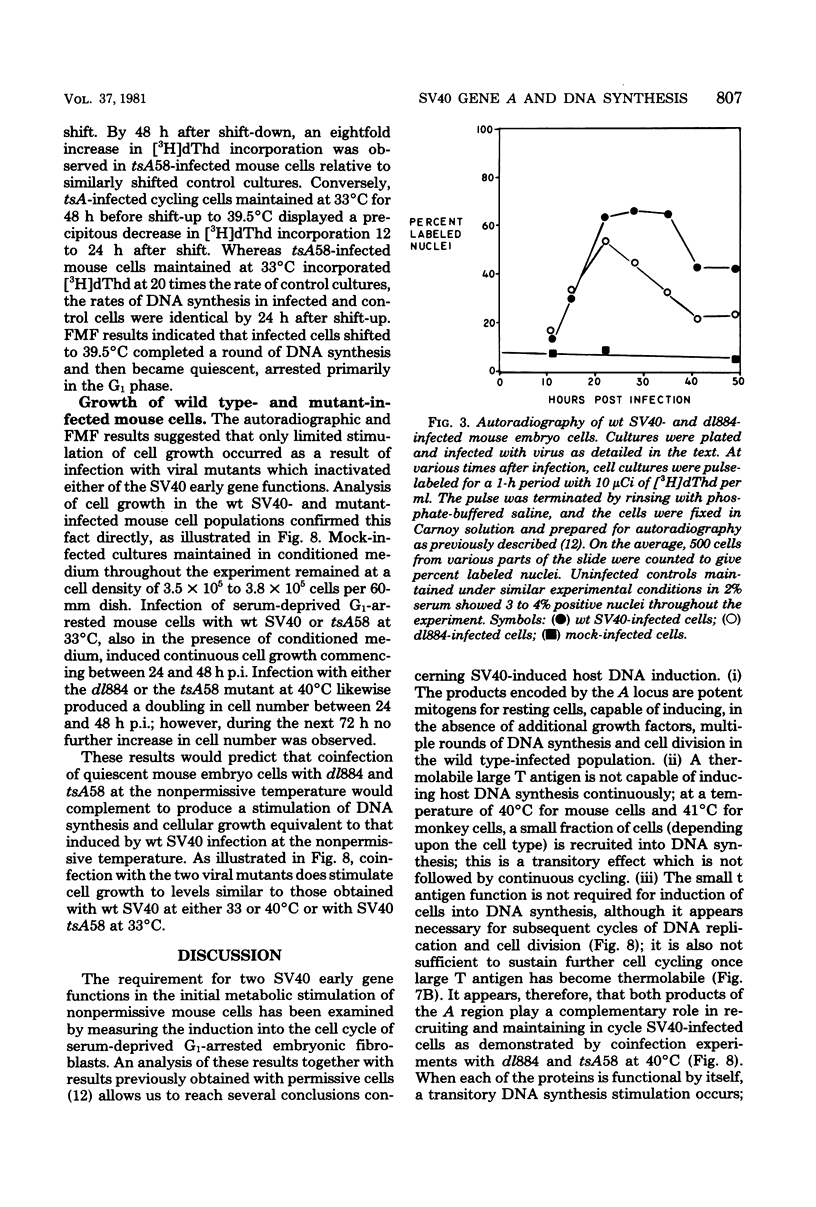
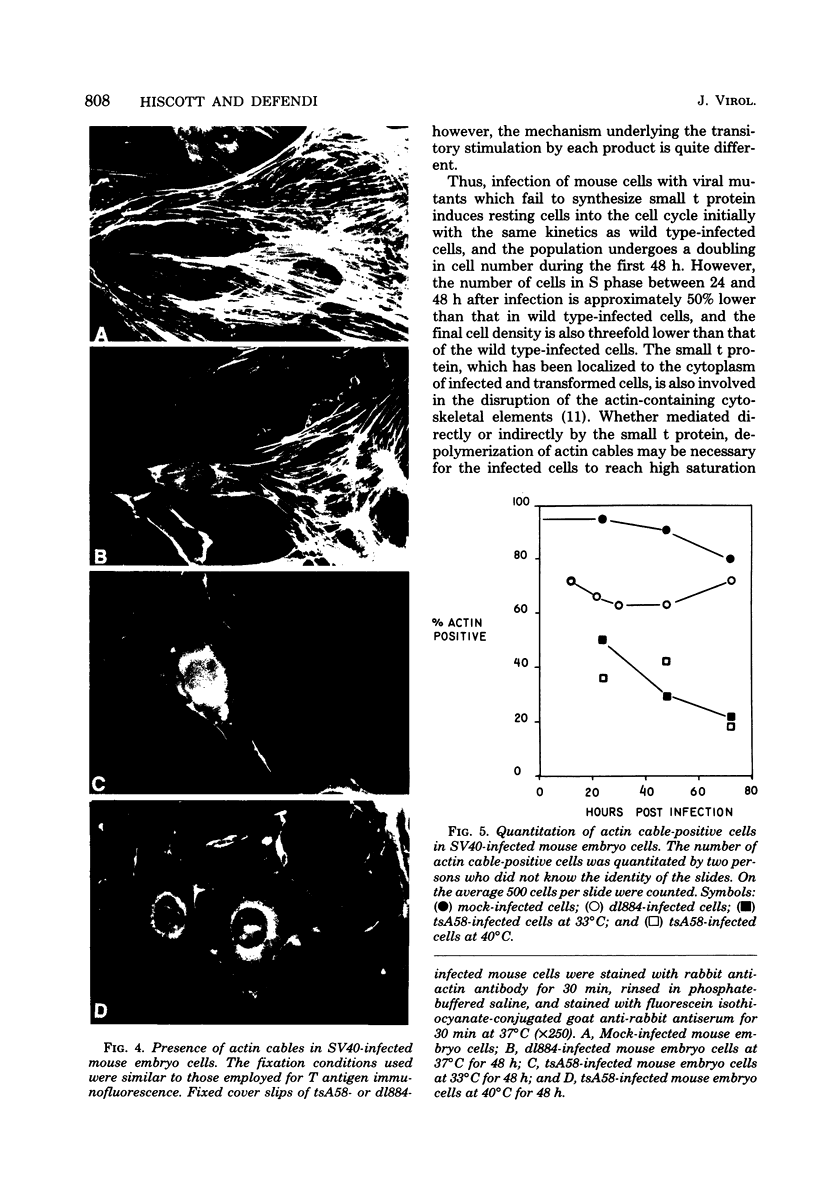
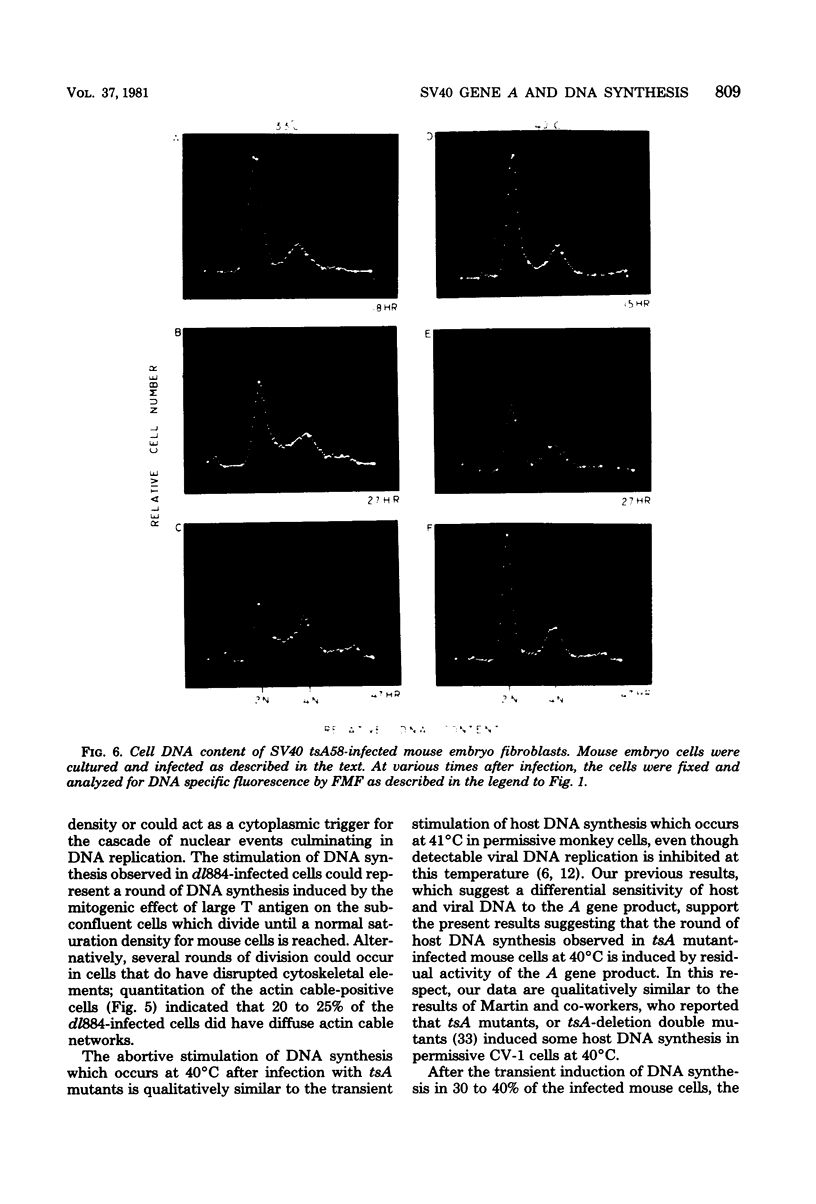
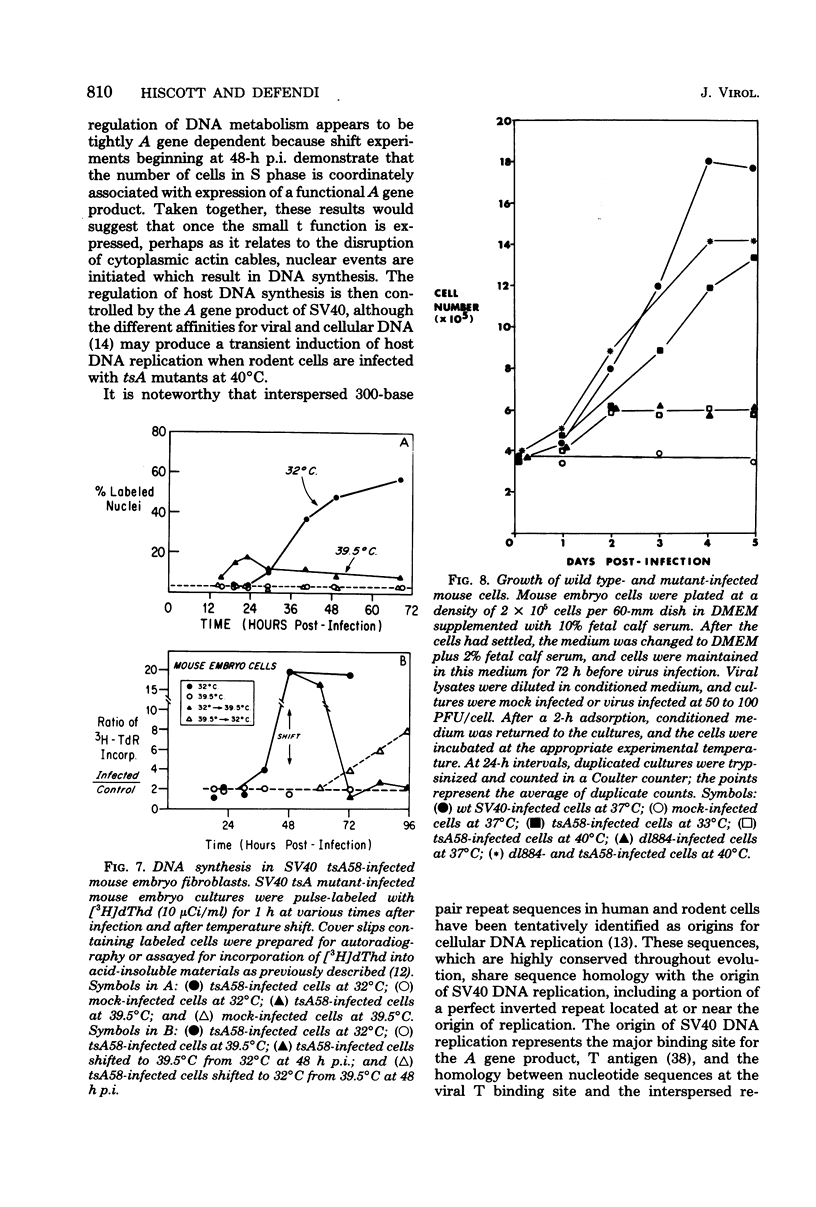
The stimulation of host macromolecular synthesis and induction into the cell cycle of serum-deprived G0-G1-arrested mouse embryo fibroblasts were examined after infection of resting cells with wild-type simian virus 40 or with viral mutants affecting T antigen (tsA58) or small t antigen (dl884). At various times after virus infection, cell cultures were analyzed for DNA synthesis by autoradiography and flow microfluorimetry. Whereas mock-infected cultured remained quiescent and displayed either a 2N DNA content (80%) or a 4N DNA content (15%), mouse cells infected with wild-type simian virus 40, tsA58 at 33 degrees C, or dl884 were induced into active cell cycling at approximately 18 h postinfection. Although dl884-infected mouse cells were induced to cycle initially at the same rate as wild type-infected cells, they became arrested earlier after infection and also failed to reach the saturation densities of wild-type simian virus 40-infected cells. Infection with dl884 also failed to induce loss of cytoplasmic actin cables in the majority of the infected cell population. Mouse cells infected with tsA58 and maintained at 39.5 degrees C showed a transient burst of DNA synthesis as reflected by changes in cell DNA content and an increase in the number of labeled nuclei during the first 24 h postinfection; however, after the abortive stimulation of DNA synthesis at 39.5 degrees C shift experiments demonstrated that host DNA replication was regulated by a functional A gene product. It is concluded that both products of the early region of simian virus 40 DNA play a complementary role in recruiting and maintaining simian virus 40-infected cells in the cell cycle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. L., Chang C., Mora P. T., Martin R. G. Expression and thermal stability of simian virus 40 tumor-specific transplantation antigen and tumor antigen in wild type- and tsA mutant-transformed cells. J Virol. 1977 Feb;21(2):459–467. doi: 10.1128/jvi.21.2.459-467.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: temperature sensitivity of the transformed phenotype and retransofrmation by wild-type virus. J Virol. 1978 Mar;25(3):860–870. doi: 10.1128/jvi.25.3.860-870.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. S., Stadtman T. C. Selenium-containing tRNAs from Clostridium sticklandii: cochromatography of one species with L-prolyl-tRNA. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1403–1407. doi: 10.1073/pnas.77.3.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. DNA infectivity and the induction of host DNA synthesis with temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):145–150. doi: 10.1128/jvi.15.1.145-150.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Cole C. N., Smith A. E., Paucha E., Tegtmeyer P., Rundell K., Berg P. Organization and expression of early genes of simian virus 40. Proc Natl Acad Sci U S A. 1978 Jan;75(1):117–121. doi: 10.1073/pnas.75.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo A. B., Jay G., Appella E., Dubois G. C., Law L. W., Old L. J. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc Natl Acad Sci U S A. 1979 May;76(5):2420–2424. doi: 10.1073/pnas.76.5.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck M. M., Benjamin T. L. Comparisons of two early gene functions essential for transformation in polyoma virus and SV-40. Virology. 1979 Jul 15;96(1):205–228. doi: 10.1016/0042-6822(79)90185-5. [DOI] [PubMed] [Google Scholar]
- Fried J., Perez A. G., Clarkson B. D. Rapid hypotonic method for flow cytofluorometry of monolayer cell cultures. Some pitfalls in staining and data analysis. J Histochem Cytochem. 1978 Nov;26(11):921–933. doi: 10.1177/26.11.82573. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Rifkin D. B., Topp W. C. Requirement for the large T and small T proteins of SV40 in the maintenance of the transformed state. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):325–331. doi: 10.1101/sqb.1980.044.01.037. [DOI] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Tjian R., Topp W. C. Simian virus 40 small-t protein is required for loss of actin cable networks in rat cells. J Virol. 1980 Mar;33(3):1182–1191. doi: 10.1128/jvi.33.3.1182-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J. B., Defendi V. Simian virus 40 gene A regulation of cellular DNA synthesis. I. In permissive cells. J Virol. 1979 May;30(2):590–599. doi: 10.1128/jvi.30.2.590-599.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian E. W., Matter J. M., Léonard N., Weil R. Simian virus 40 and polyoma virus stimulate overall cellular RNA and protein synthesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1476–1480. doi: 10.1073/pnas.77.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Gruss P., Dhar R., Lai C. J. Processing and expression of early SV40 mRNA: a role for RNA conformation in splicing. Cell. 1979 Sep;18(1):85–92. doi: 10.1016/0092-8674(79)90356-8. [DOI] [PubMed] [Google Scholar]
- Kimura G., Dulbecco R. A temperature-sensitive mutant of simian virus 40 affecting transforming ability. Virology. 1973 Apr;52(2):529–534. doi: 10.1016/0042-6822(73)90348-6. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A. Initiation and maintenance of cell transformation by simian virus 40: a viral genetic property. Proc Natl Acad Sci U S A. 1975 Feb;72(2):673–677. doi: 10.1073/pnas.72.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Maltzman W., Levine A. J. The SV40 A gene product is required for the production of a 54,000 MW cellular tumor antigen. Virology. 1979 Oct 30;98(2):308–318. doi: 10.1016/0042-6822(79)90554-3. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Oppenheim A. Initiation points for DNA replication in nontransformed and simian virus 40-transformed Chinese hamster lung cells. Cell. 1977 Aug;11(4):859–869. doi: 10.1016/0092-8674(77)90297-5. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A., Vembu D. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell. 1979 Jul;17(3):635–643. doi: 10.1016/0092-8674(79)90271-x. [DOI] [PubMed] [Google Scholar]
- Melero J. A., Tur S., Carroll R. B. Host nuclear proteins expressed in simian virus 40-transformed and -infected cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):97–101. doi: 10.1073/pnas.77.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C., Graessmann A., Graessmann M. Mapping of early SV40-specific functions by microinjection of different early viral DNA fragments. Cell. 1978 Oct;15(2):579–585. doi: 10.1016/0092-8674(78)90026-0. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucha E., Smith A. E. The sequences between 0.59 and 0.54 map units on SV40 DNA code for the unique region of small t antigen. Cell. 1978 Nov;15(3):1011–1020. doi: 10.1016/0092-8674(78)90285-4. [DOI] [PubMed] [Google Scholar]
- Prives C., Gilboa E., Revel M., Winocour E. Cell-free translation of simian virus 40 early messenger RNA coding for viral T-antigen. Proc Natl Acad Sci U S A. 1977 Feb;74(2):457–461. doi: 10.1073/pnas.74.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell K., Cox J. Simian virus 40 t antigen affects the sensitivity of cellular DNA synthesis to theophylline. J Virol. 1979 Apr;30(1):394–396. doi: 10.1128/jvi.30.1.394-396.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A., Brockman W. W., Nathans D. Biological activities of deletion mutants of simian virus 40. Virology. 1976 Dec;75(2):319–334. doi: 10.1016/0042-6822(76)90031-3. [DOI] [PubMed] [Google Scholar]
- Setlow V. P., Persico-Dilauro M., Edwards C. A., Martin R. G. The isolation of SV40 tsA/deletion, double mutants and the induction of host DNA synthesis. Virology. 1980 Feb;101(1):250–260. doi: 10.1016/0042-6822(80)90500-0. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Ozer H. L. Temperature-sensitive mutants of simian virus 40: infection of permissive cells. J Virol. 1971 Oct;8(4):516–524. doi: 10.1128/jvi.8.4.516-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Weil R. Viral 'tumor antigens': A novel type of mammalian regulator protein. Biochim Biophys Acta. 1978 Nov 17;516(3):301–388. doi: 10.1016/0304-419x(78)90012-4. [DOI] [PubMed] [Google Scholar]