Abstract
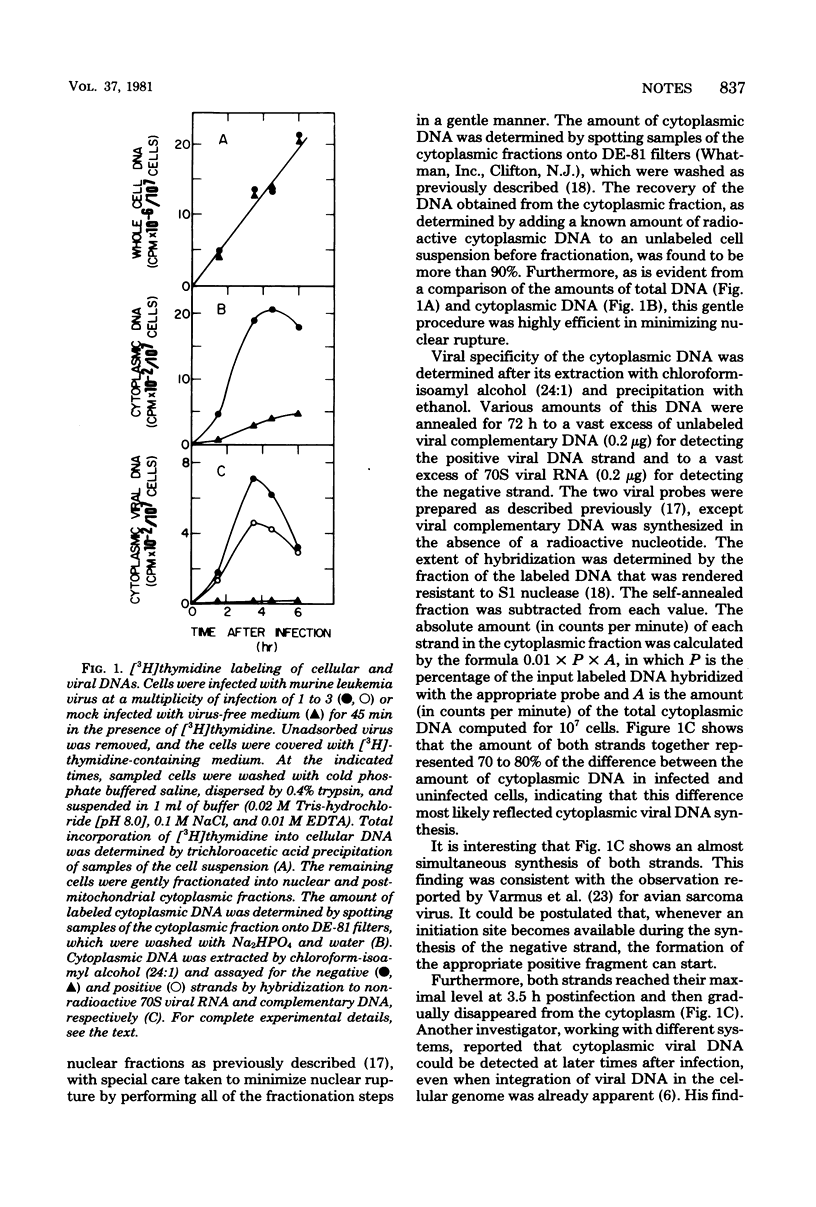
Cytoplasmic viral DNA synthesis can be followed efficiently by [3H]thymidine labeling of cells exogenously infected with Moloney murine leukemia virus. Both the negative and the positive strands of viral DNA reached their maximal level in the cytoplasm at 3.5 h postinfection. Interferon treatment before infection markedly reduced the amount of viral DNA formed during the first 3.5 h, but led to a second major wave of viral DNA synthesis, peaking at 7.5 h postinfection. No such late cytoplasmic DNA synthesis occurred in the untreated control. Inhibition of protein synthesis by cycloheximide, on the other hand, stimulated cytoplasmic viral DNA synthesis during the first 3.5 h.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud M., Shoor R., Salzberg S. Adsorption, penetration, and uncoating of murine leukemia virus studied by using its reverse transcriptase. J Virol. 1979 Apr;30(1):32–37. doi: 10.1128/jvi.30.1.32-37.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboud M., Shoor R., Salzberg S. Effect of interferon on exogenous murine leukemia virus infection. Virology. 1978 Jan;84(1):134–141. doi: 10.1016/0042-6822(78)90225-8. [DOI] [PubMed] [Google Scholar]
- Aboud M., Weiss O., Salzberg S. Rapid quantitation of interferon with chronically oncornavirus-producing cells. Infect Immun. 1976 Jun;13(6):1626–1632. doi: 10.1128/iai.13.6.1626-1632.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., Baluda M. A. Synthesis of avian oncornavirus DNA in infected chicken cells. J Virol. 1974 May;13(5):1005–1013. doi: 10.1128/jvi.13.5.1005-1013.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiau A. Effect of interferon on RNA tumor viruses. Tex Rep Biol Med. 1977;35:406–419. [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Dales S., Hanafusa H. Penetration and intracellular release of the genomes of avian RNA tumor viruses. Virology. 1972 Nov;50(2):440–458. doi: 10.1016/0042-6822(72)90396-0. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Antiviral activity of interferons. Bacteriol Rev. 1977 Sep;41(3):543–567. doi: 10.1128/br.41.3.543-567.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallis B. M., Eisenman R. N., Diggelmann H. Synthesis of the precursor to avian RNA tumor virus internal structural proteins early after infection. Virology. 1976 Oct 15;74(2):302–313. doi: 10.1016/0042-6822(76)90337-8. [DOI] [PubMed] [Google Scholar]
- Gianni A. M., Smotkin D., Weinberg R. A. Murine leukemia virus: detection of unintegrated double-stranded DNA forms of the provirus. Proc Natl Acad Sci U S A. 1975 Feb;72(2):447–451. doi: 10.1073/pnas.72.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschka P. V. Analysis of nucleotide pools in animal cells. Methods Cell Biol. 1973;7:361–462. doi: 10.1016/s0091-679x(08)61787-2. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of Fv-1 gene product on proviral DNA formation and integration in cells infected with murine leukemia viruses. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2236–2240. doi: 10.1073/pnas.73.7.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger G. G., Klein R., Ling H. P., Gilden R. V., Hatanaka M. Kinetics of murine type C virus-specific DNA synthesis newly infected cells. J Virol. 1975 Oct;16(4):824–831. doi: 10.1128/jvi.16.4.824-831.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Gilden R. V. Electron microscopic studies of tumor viruses. I. Entry of murine leukemia virus into mouse embryo fibroblasts. J Virol. 1971 Mar;7(3):395–406. doi: 10.1128/jvi.7.3.395-406.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitha P. M., Rowe W. P., Oxman M. N. Effect of interferon on exogenous, endogenous, and chroniv murine leukemia virus infection. Virology. 1976 Apr;70(2):324–338. doi: 10.1016/0042-6822(76)90275-0. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Salzberg S., Bakhanashvili M., Aboud M. Effect of interferon on mouse cells chronically infected with murine leukaemia virus: kinetic studies on virus production and virus RNA synthesis. J Gen Virol. 1978 Jul;40(1):121–130. doi: 10.1099/0022-1317-40-1-121. [DOI] [PubMed] [Google Scholar]
- Salzberg S., Levi Z., Aboud M., Goldberger A. Isolation and characterization of DNA-DNA and DNA-RNA. Biochemistry. 1977 Jan 11;16(1):25–29. doi: 10.1021/bi00620a004. [DOI] [PubMed] [Google Scholar]
- Salzberg S., Robin M. S., Green M. A possible requirement for protein synthesis early in the infectious cycle of the murine sarcoma-leukemia virus. Virology. 1977 Jan;76(1):341–351. doi: 10.1016/0042-6822(77)90307-5. [DOI] [PubMed] [Google Scholar]
- Shurtz R., Dolev S., Aboud M., Salzberg S. Viral genome RNA serves as messenger early in the infectious cycle of murine leukemia virus. J Virol. 1979 Sep;31(3):668–676. doi: 10.1128/jvi.31.3.668-676.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Allfrey V. G., Hanafusa H. Microinjection analysis of envelope-glycoprotein messenger activities of avian leukosis viral RNAs. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1614–1618. doi: 10.1073/pnas.74.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Guntaka R. V., Fan W. J., Heasley S., Bishop J. M. Synthesis of viral DNA in the cytoplasm of duck embryo fibroblasts and in enucleated cells after infection by avian sarcoma virus. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3874–3878. doi: 10.1073/pnas.71.10.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]


