Abstract
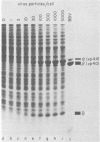
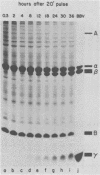
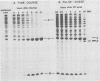
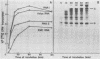
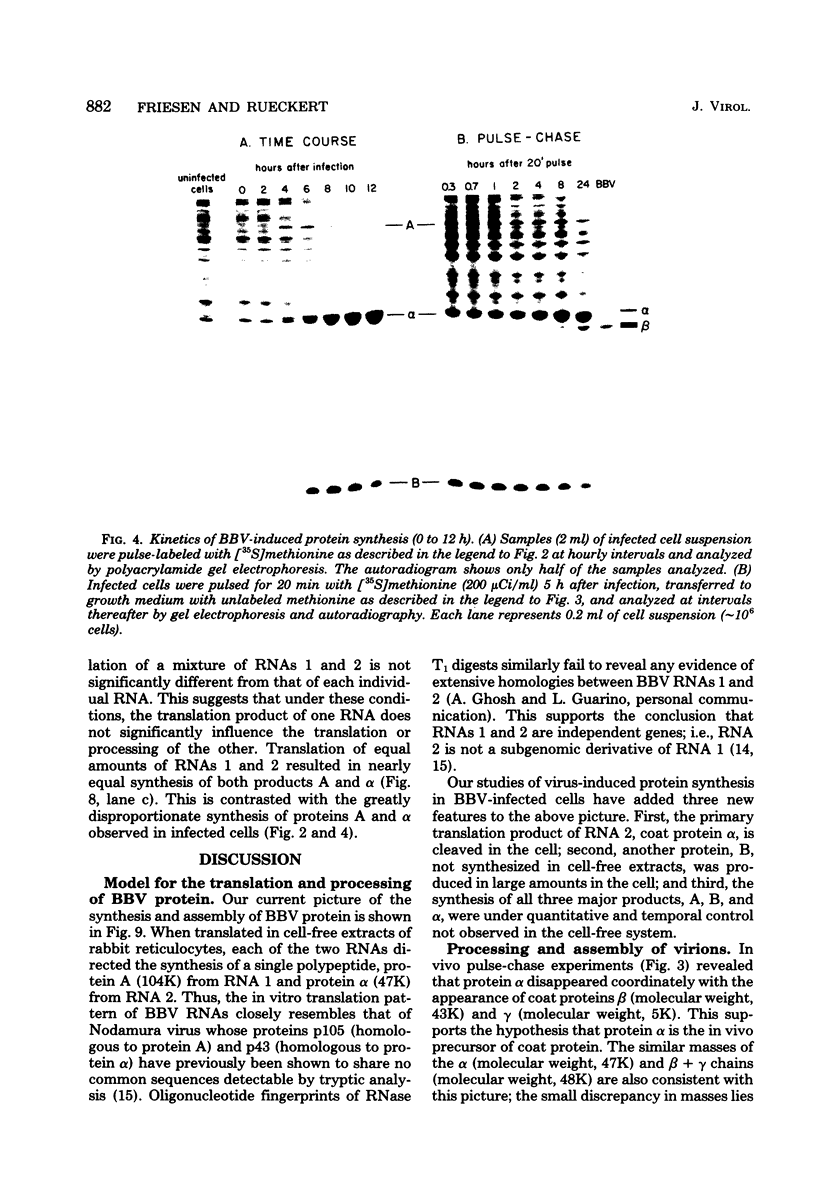
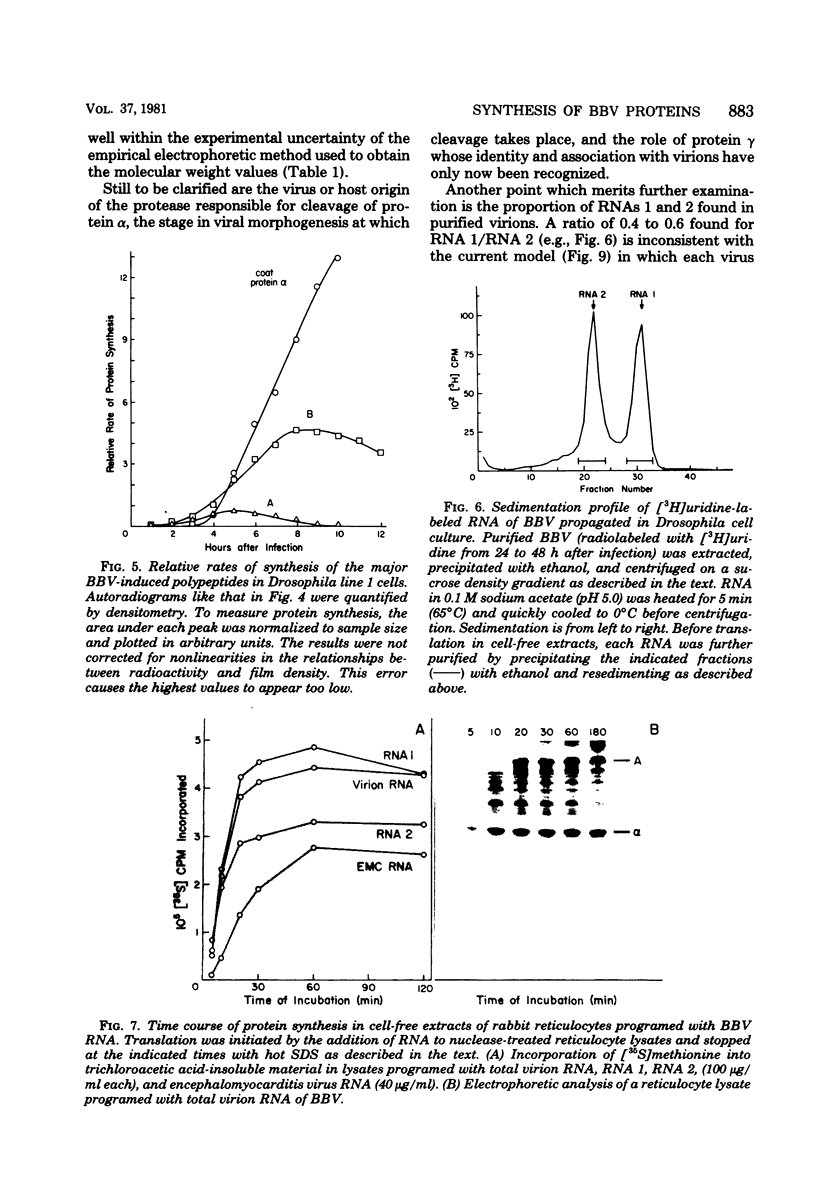
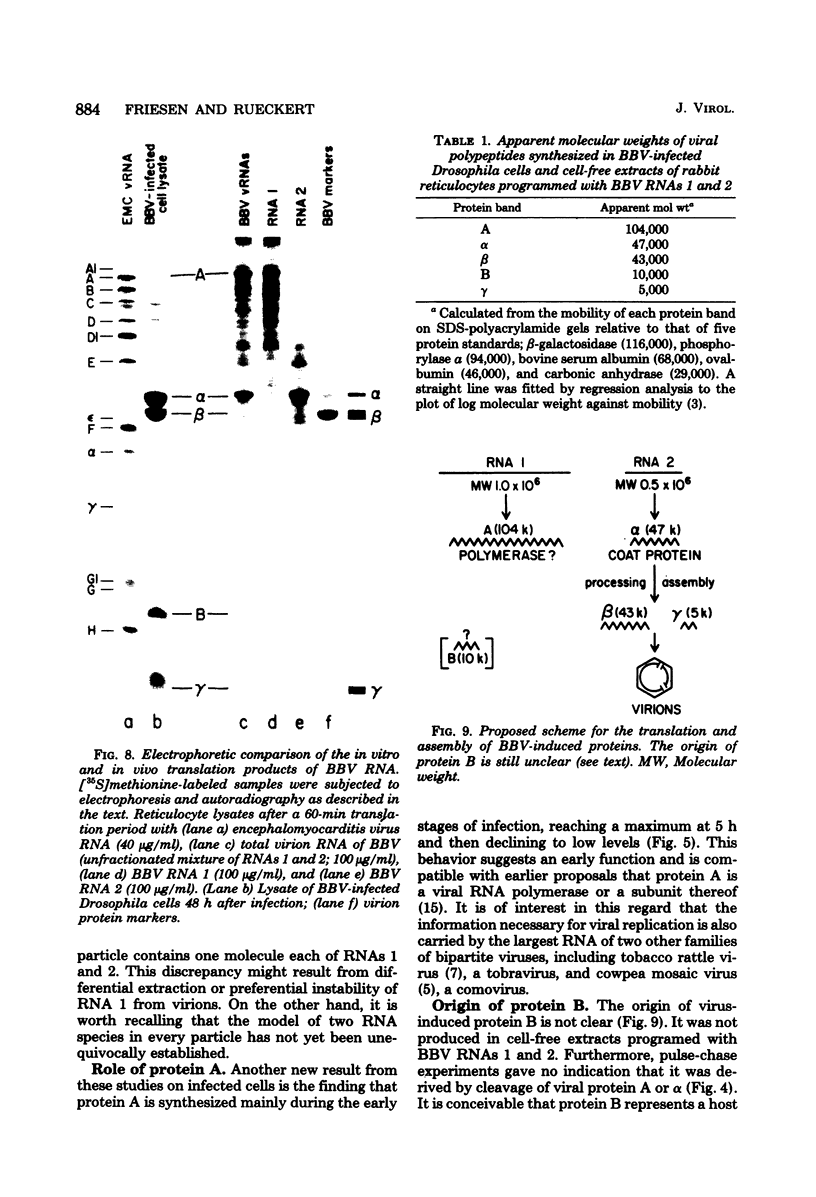
Black beetle virus is an insect virus with a split genome consisting of two single-stranded, messenger-active RNA molecules with molecular weights of 1.0 × 106 (RNA 1) and 0.5 × 106 (RNA 2), respectively. Virions contained two proteins, β with a molecular weight of 43,000 (43K) and γ (5K), and traces of a third protein, α (47K). When translated in cell-free extracts of rabbit reticulocytes, RNA 1 directed the synthesis of protein A (104K), whereas RNA 2 synthesized protein α. The in vitro translation efficiency of the two RNAs was roughly equal. Infection of cultured Drosophila cells induced the synthesis of five new proteins: A, α, β, γ, and B (10K), detected by autoradiography of polyacrylamide gels after electrophoresis of extracts from [35S]methionine-labeled cultures. All but protein γ could also be detected by staining with Coomassie brilliant blue, indicating vigorous synthesis of viral proteins. Pulse-chase experiments in infected cells revealed the disappearance of protein α and the coordinate appearance of proteins β and γ, supporting an earlier proposal that coat protein of mature virions is made by cleavage of precursor α. Proteins A and B were stable in such pulse-chase experiments. The three classes of virus-induced proteins, represented by A, B, and α, were synthesized in markedly different amounts and with different kinetics. Synthesis of proteins A and B peaked early in infection and then declined, whereas synthesis of coat protein precursor α peaked much later. These results suggest that RNA 1 controls early replication functions via protein A (and also possibly protein B), whereas RNA 2 controls synthesis of coat protein required later for virion assembly.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Friesen P., Scotti P., Longworth J., Rueckert R. Black beetle virus: propagation in Drosophila line 1 cells and an infection-resistant subline carrying endogenous black beetle virus-related particles. J Virol. 1980 Sep;35(3):741–747. doi: 10.1128/jvi.35.3.741-747.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. R., Hunt T., Knowland J., Zimmern D. Messenger RNA for the coat protein of tobacco mosaic virus. Nature. 1976 Apr 29;260(5554):759–764. doi: 10.1038/260759a0. [DOI] [PubMed] [Google Scholar]
- Lister R. M. Tobacco rattle, NETU, viruses in relation to functional heterogeneity in plant viruses. Fed Proc. 1969 Nov-Dec;28(6):1875–1889. [PubMed] [Google Scholar]
- Longworth J. F., Carey G. P. A small RNA virus with a divided genome from Heteronychus arator (F.) [Coleoperai Scarabaeidae]. J Gen Virol. 1976 Oct;33(1):31–40. doi: 10.1099/0022-1317-33-1-31. [DOI] [PubMed] [Google Scholar]
- Longworth J. F. Small isometric viruses of invertebrates. Adv Virus Res. 1978;23:103–157. doi: 10.1016/s0065-3527(08)60099-8. [DOI] [PubMed] [Google Scholar]
- Medappa K. C., McLean C., Rueckert R. R. On the structure of rhinovirus 1A. Virology. 1971 May;44(2):259–270. doi: 10.1016/0042-6822(71)90258-3. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Scherer W. F., Harrison A. K., Dunne H. W., Gary G. W., Jr Characterization of Nodamura virus, an arthropod transmissible picornavirus. Virology. 1970 Apr;40(4):1008–1021. doi: 10.1016/0042-6822(70)90147-9. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Brown F. Further physicochemical characterization of Nodamura virus. Evidence that the divided genome occurs in a single component. J Gen Virol. 1978 Jan;38(1):83–95. doi: 10.1099/0022-1317-38-1-83. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Synthesis and proteolytic processing of cowpea mosaic virus proteins in reticulocyte lysates. Virology. 1979 Jul 30;96(2):463–477. doi: 10.1016/0042-6822(79)90104-1. [DOI] [PubMed] [Google Scholar]
- Pleij C. W., Neeleman A., van Vloten-Doting L., Bosch L. Translation of turnip yellow mosaic virus RNA in vitro: a closed and an open coat protein cistron. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4437–4441. doi: 10.1073/pnas.73.12.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER I. DIFFERENTIATION OF LARVAL DROSOPHILA EYE-ANTENNAL DISCS IN VITRO. J Exp Zool. 1964 Jun;156:91–103. doi: 10.1002/jez.1401560107. [DOI] [PubMed] [Google Scholar]
- Scherer W. F., Hurlbut H. S. Nodamura virus from Japan: a new and unusual arbovirus resistant to diethyl ether and chloroform. Am J Epidemiol. 1967 Sep;86(2):271–285. doi: 10.1093/oxfordjournals.aje.a120737. [DOI] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972 Apr;27(2):353–365. [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Kew O., Pallansch M., Rueckert R., Kaesberg P. Cell-free synthesis and processing of the proteins of poliovirus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5807–5811. doi: 10.1073/pnas.75.12.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]