Abstract
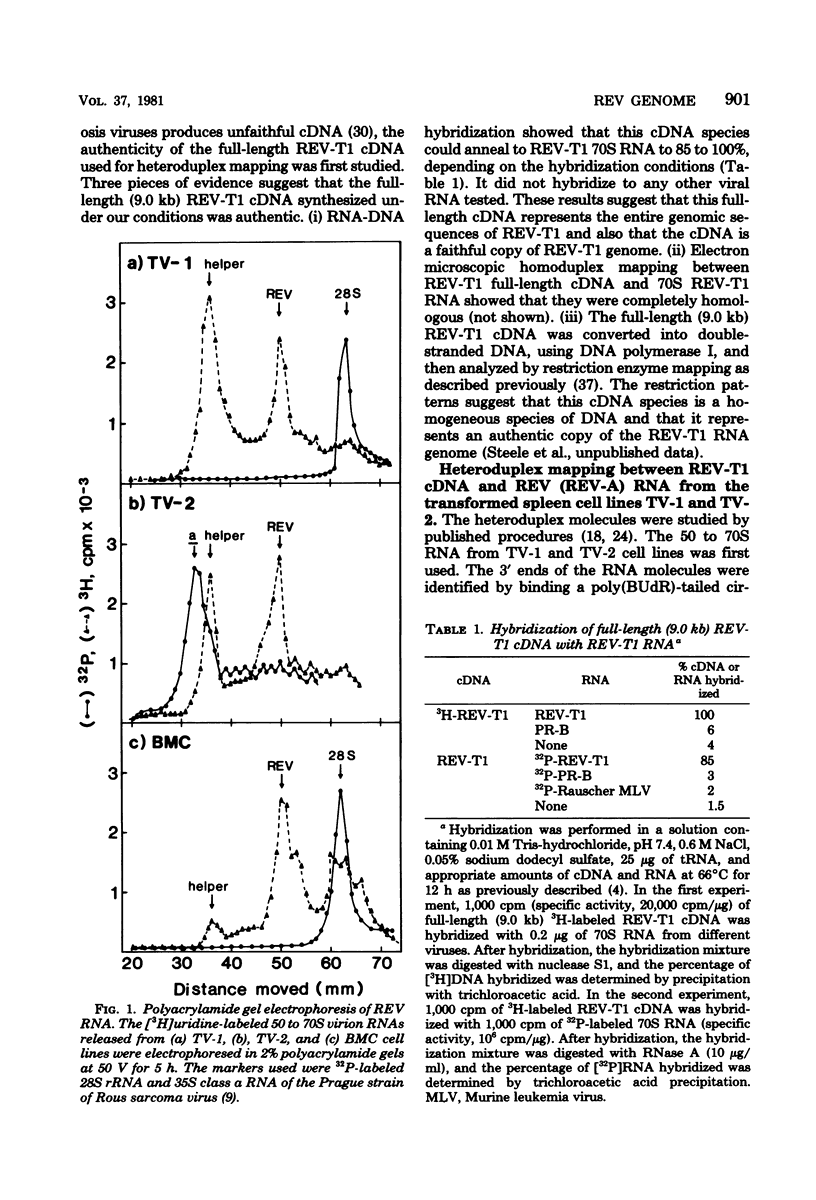
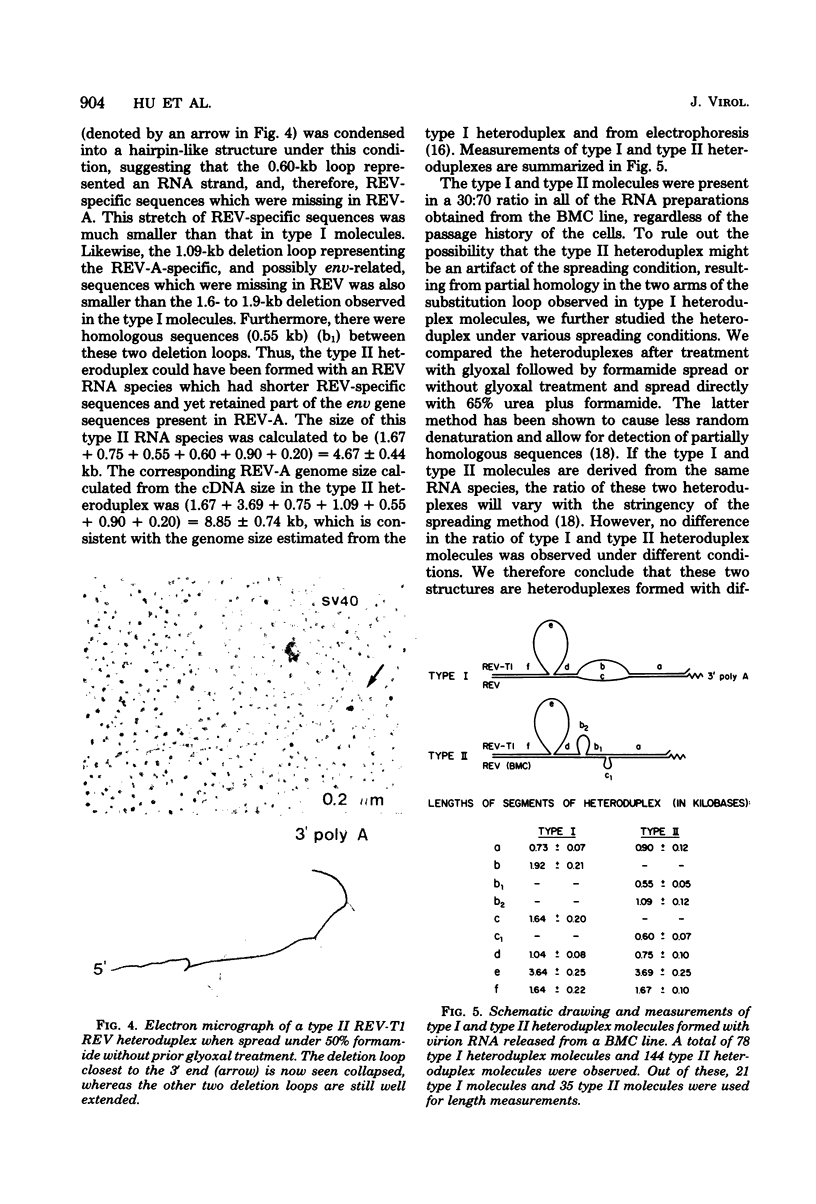
The genome structure of defective, oncogenic avian reticuloendotheliosis virus (REV) was studied by heteroduplex mapping between the full-length complementary DNA of the helper virus REV-T1 and the 30S REV RNA. The REV genome (5.5 kilobases) had a deletion of 3.69 kilobases in the gag-pol region, confirming the genetic defectiveness of REV. In addition, REV lacked the sequences corresponding to the env gene but contained, instead, a contiguous stretch (1.6 to 1.9 kilobases) of the specific sequences presumably related to viral oncogenicity. Unlike those of other avian acute leukemia viruses, the transformation-specific sequences of REV were not contiguous with the gag-pol deletion. Thus, REV has a genome structure similar to that of a defective mink cell focus-inducing virus or a defective murine sarcoma virus. An additional class of heteroduplex molecules containing the gag-pol deletion and two other smaller deletion loops was observed. These molecules probably represented recombinants between the oncogenic REV and its helper virus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender W., Davidson N. Mapping of poly(A) sequences in the electron microscope reveals unusual structure of type C oncornavirus RNA molecules. Cell. 1976 Apr;7(4):595–607. doi: 10.1016/0092-8674(76)90210-5. [DOI] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Bosselman R. A., Van Griensven L. J., Vogt M., Verma I. M. Genome organization of retroviruses IX. Analysis of the genomes of Friend spleen focus-forming (F-SFFV) and helper murine leukemia viruses by heteroduplex-formation. Virology. 1980 Apr 15;102(1):234–239. doi: 10.1016/0042-6822(80)90088-4. [DOI] [PubMed] [Google Scholar]
- Breitman M. L., Lai M. M., Vogt P. K. The genomic RNA of avian reticuloendotheliosis virus REV. Virology. 1980 Jan 30;100(2):450–461. doi: 10.1016/0042-6822(80)90535-8. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Champion M., Chabot F. Nucleotide sequence relationships between the genomes of an endogenous and an exogenous avian tumor virus. J Virol. 1978 Dec;28(3):972–991. doi: 10.1128/jvi.28.3.972-991.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler S., Ruta M., Murray M. J., Kabat D. Glycoprotein encoded by the Friend spleen focus-forming virus. J Virol. 1979 May;30(2):564–575. doi: 10.1128/jvi.30.2.564-575.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Vogt P. K. The RNA of avian acute leukemia virus MC29. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4320–4324. doi: 10.1073/pnas.74.10.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. RNA species obtained from clonal lines of avian sarcoma and from avian leukosis virus. Virology. 1973 Jul;54(1):207–219. doi: 10.1016/0042-6822(73)90130-x. [DOI] [PubMed] [Google Scholar]
- Franklin R. B., Kang C. Y., Min-Min Wan K., Bose H. R., Jr Transformation of chick embryo fibroblasts by reticuloendotheliosis virus. Virology. 1977 Dec;83(2):313–321. doi: 10.1016/0042-6822(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Franklin R. B., Maldonado R. L., Bose H. R. Isolation and characterization of reticuloendotheliosis virus transformed bone marrow cells. Intervirology. 1974;3(5-6):342–352. doi: 10.1159/000149771. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Rice N. R., Gilden R. V. Avian reticuloendotheliosis virus: characterization of the high-molecular-weight viral RNA in transforming and helper virus populations. J Virol. 1980 Jun;34(3):743–751. doi: 10.1128/jvi.34.3.743-751.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. S., Wade E., Rucker E., Baxter-Gabbard K. L., Levine A. S., Friis R. R. A study of the relationship of reticuloendotheliosis virus to the avian leukosis-sarcoma complex of viruses. Virology. 1973 Jun;53(2):287–299. doi: 10.1016/0042-6822(73)90206-7. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Royer-Pokora B., Graf T. Defectiveness of avian erythroblastosis virus: synthesis of a 75K gag-related protein. Virology. 1979 Jan 15;92(1):31–45. doi: 10.1016/0042-6822(79)90212-5. [DOI] [PubMed] [Google Scholar]
- Hoelzer J. D., Franklin R. B., Bose H. R., Jr Transformation by reticuloendotheliosis virus: development of a focus assay and isolation of a nontransforming virus. Virology. 1979 Feb;93(1):20–30. doi: 10.1016/0042-6822(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Hoelzer J. D., Lewis R. B., Wasmuth C. R., Bose H. R., Jr Hematopoietic cell transformation by reticuloendotheliosis virus: characterization of the genetic defect. Virology. 1980 Jan 30;100(2):462–474. doi: 10.1016/0042-6822(80)90536-x. [DOI] [PubMed] [Google Scholar]
- Hu S. F., Lai M. M., Vogt P. K. Characterization of the env gene in avian oncoviruses by heteroduplex mapping. J Virol. 1978 Sep;27(3):667–676. doi: 10.1128/jvi.27.3.667-676.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. S., Lai M. M., Vogt P. K. Genome of avian myelocytomatosis virus MC29: analysis by heteroduplex mapping. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1265–1268. doi: 10.1073/pnas.76.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. S., Moscovici C., Vogt P. K. The defectiveness of Mill Hill 2, a carcinoma-inducing avian oncovirus. Virology. 1978 Aug;89(1):162–178. doi: 10.1016/0042-6822(78)90049-1. [DOI] [PubMed] [Google Scholar]
- Hu S., Davidson N. A heteroduplex study of the sequence relationships between the RNAs of M-MSV and M-MLV. Cell. 1977 Mar;10(3):469–477. doi: 10.1016/0092-8674(77)90034-4. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Hu S., Knight C. A., Davidson N. Heteroduplex analysis of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1977 Feb;74(2):477–481. doi: 10.1073/pnas.74.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L. H., Rufner R., Sevoian M. Isolation and development of a reticuloendotheliosis virus-transformed lymphoblastoid cell line from chicken spleen cells. Infect Immun. 1979 Aug;25(2):694–701. doi: 10.1128/iai.25.2.694-701.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Bailey J. M., Davidson N., Nicolson M. O., McAllister R. M. Structure, subunit composition, and molecular weight of RD-114 RNA. J Virol. 1975 Aug;16(2):397–411. doi: 10.1128/jvi.16.2.397-411.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Hu S. S. In vitro synthesis and characterisation of full- and half-genome length complementary DNA from avian oncoviruses. Nature. 1978 Feb 2;271(5644):481–483. doi: 10.1038/271481a0. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Hu S. S., Vogt P. K. Avian erythroblastosis virus: transformation-specific sequences form a contiguous segment of 3.25 kb located in the middle of the 6-kb genome. Virology. 1979 Sep;97(2):366–377. doi: 10.1016/0042-6822(79)90347-7. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Hu S. S., Vogt P. K. Occurrence of partial deletion and substitution of the src gene in the RNA genome of avian sarcoma virus. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4781–4785. doi: 10.1073/pnas.74.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M. Phosphoproteins of Rous sarcoma viruses. Virology. 1976 Oct 15;74(2):287–301. doi: 10.1016/0042-6822(76)90336-6. [DOI] [PubMed] [Google Scholar]
- Maldonado R. L., Bose H. R. Relationship of reticuloendotheliosis virus to the avian tumor viruses: nucleic acid and polypeptide composition. J Virol. 1973 May;11(5):741–747. doi: 10.1128/jvi.11.5.741-747.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani A., Temin H. M. Purification and properties of spleen necrosis virus DNA polymerase. J Virol. 1975 Oct;16(4):797–806. doi: 10.1128/jvi.16.4.797-806.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L. D. Histopathologic and hematologic changes in moribund stages of chicks infected with T-virus. Am J Vet Res. 1967 Sep;28(126):1501–1507. [PubMed] [Google Scholar]
- Purchase H. G., Ludford C., Nazerian K., Cox H. W. A new group of oncogenic viruses: reticuloendotheliosis, chick syncytial, duck infectious anemia, and spleen necrosis viruses. J Natl Cancer Inst. 1973 Aug;51(2):489–499. [PubMed] [Google Scholar]
- Purchase H. G., Witter R. L. The reticuloendotheliosis viruses. Curr Top Microbiol Immunol. 1975;71:103–124. doi: 10.1007/978-3-642-66193-8_3. [DOI] [PubMed] [Google Scholar]
- Racevskis J., Koch G. Synthesis and processing of viral proteins in Friend erythroleukemia cell lines. Virology. 1978 Jun 15;87(2):354–365. doi: 10.1016/0042-6822(78)90140-x. [DOI] [PubMed] [Google Scholar]
- Ruscetti S. K., Linemeyer D., Feild J., Troxler D., Scolnick E. M. Characterization of a protein found in cells infected with the spleen focus-forming virus that shares immunological cross-reactivity with the gp70 found in mink cell focus-inducing virus particles. J Virol. 1979 Jun;30(3):787–798. doi: 10.1128/jvi.30.3.787-798.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Hsu T. W., Lai M. M. Restriction enzyme sites on the avian RNA tumor virus genome. J Virol. 1978 May;26(2):479–484. doi: 10.1128/jvi.26.2.479-484.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilen G. H., Zeigel R. F., Twiehaus M. J. Biological studies with RE virus (strain T) that induces reticuloendotheliosis in turkeys, chickens, and Japanese quail. J Natl Cancer Inst. 1966 Dec;37(6):731–743. [PubMed] [Google Scholar]
- Troxler D. H., Scolnick E. M. Rapid leukemia induced by cloned friend strain of replicating murine type-C virus. Association with induction of xenotropic-related RNA sequences contained in spleen focus-forming virus. Virology. 1978 Mar;85(1):17–27. doi: 10.1016/0042-6822(78)90408-7. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Spencer J. L., Okazaki W., Witter R. L., Crittenden L. B. Phenotypic mixing between reticuloendotheliosis virus and avian sarcoma viruses. Virology. 1977 Jul 1;80(1):127–135. doi: 10.1016/0042-6822(77)90385-3. [DOI] [PubMed] [Google Scholar]
- Witter R. L., Purchase H. G., Burgoyne G. H. Peripheral nerve lesions similar to those of Marek's disease in chickens inoculated with reticuloendotheliosis virus. J Natl Cancer Inst. 1970 Sep;45(3):567–577. [PubMed] [Google Scholar]
- van Griensven L. J., Vogt M. Rauscher "mink cell focus-inducing" (MCF) virus causes erythroleukemia in mice: its isolation and properties. Virology. 1980 Mar;101(2):376–388. doi: 10.1016/0042-6822(80)90451-1. [DOI] [PubMed] [Google Scholar]





