Abstract
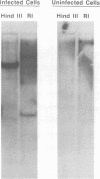
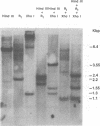
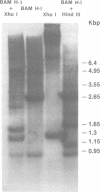
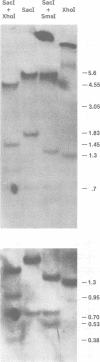
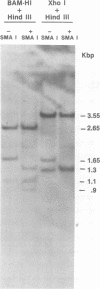
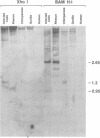
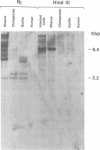
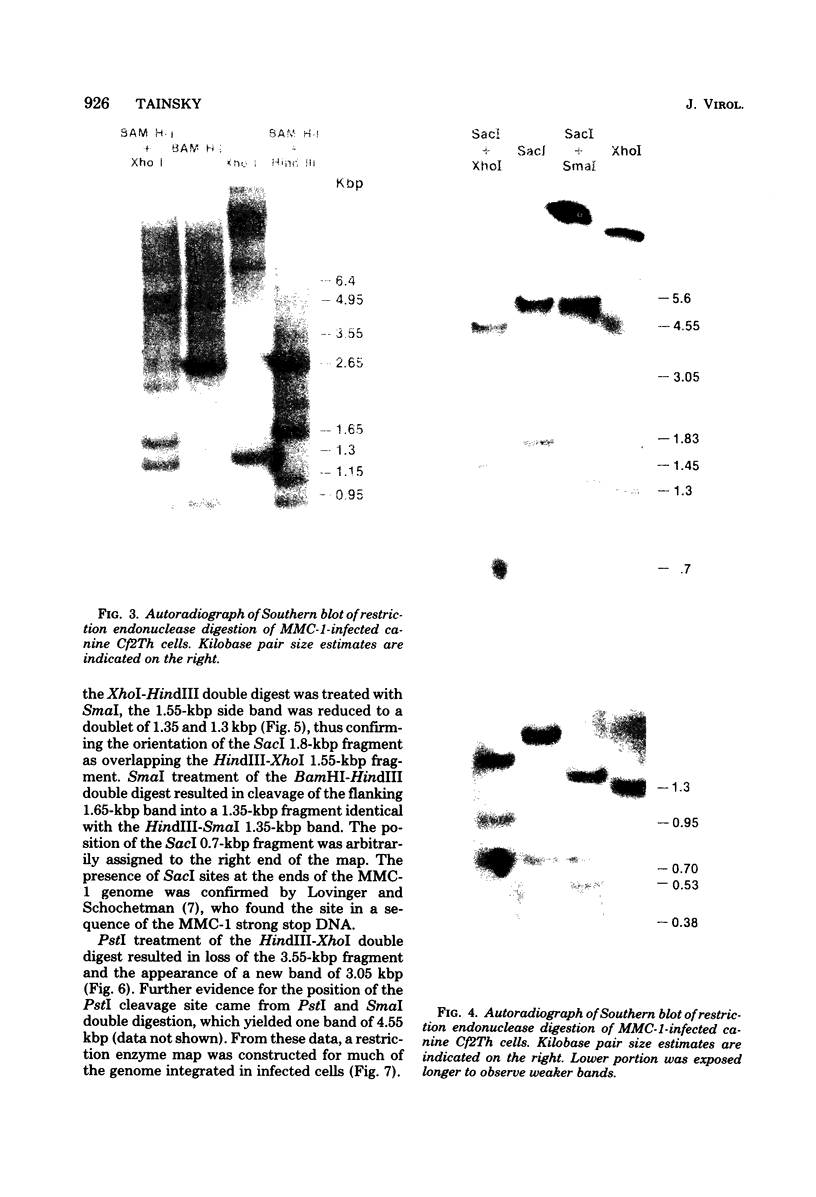
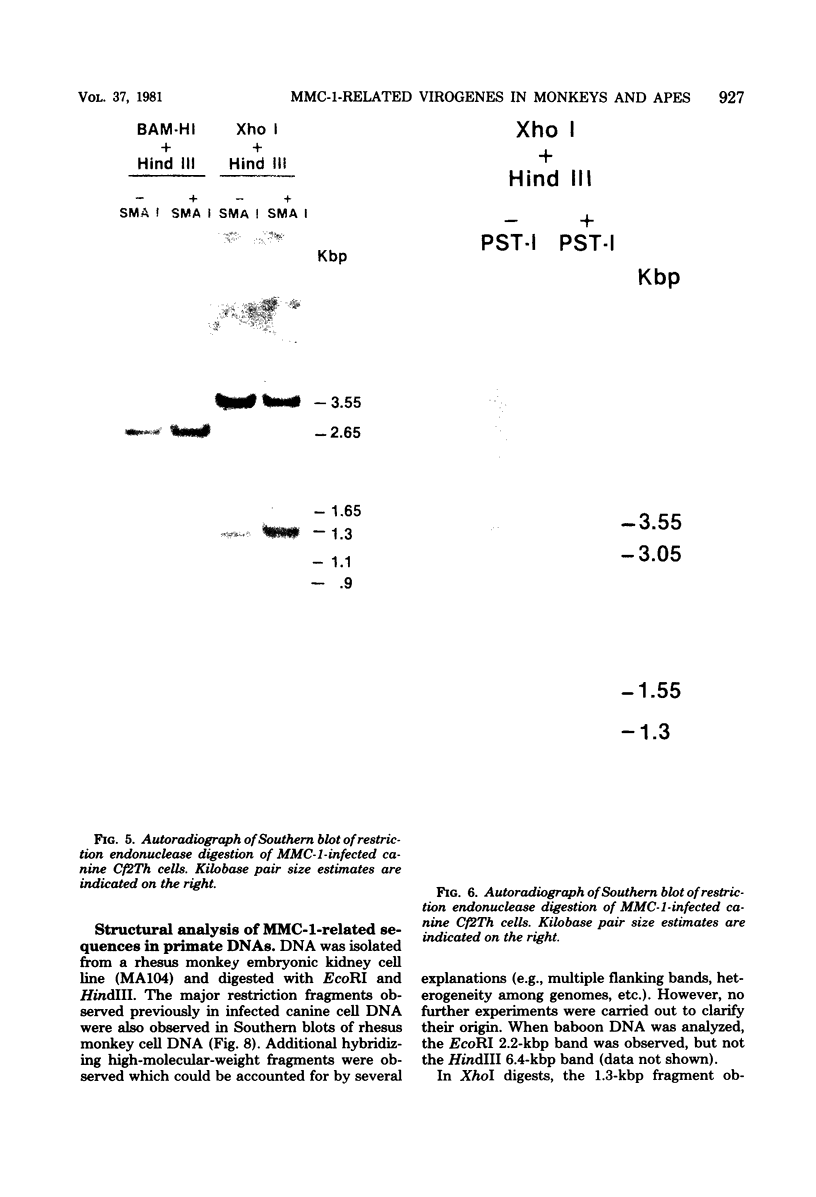
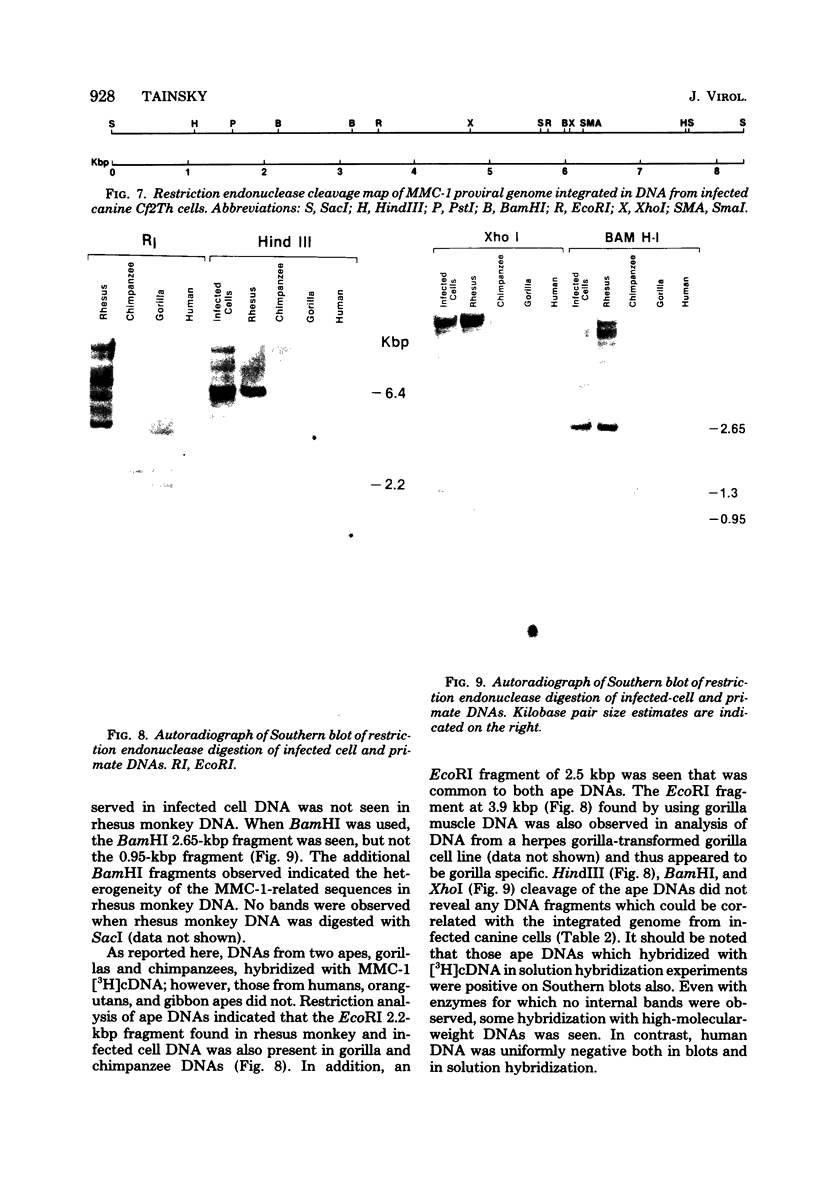
Molecular hybridization studies were carried out by using a [3H]complementary DNA (cDNA) probe to compare the endogenous type C retrovirus of rhesus monkeys (MMC-1) with other known retroviruses and related sequences in various primate DNAs. The genomic RNA of the endogenous type C retrovirus of stumptail monkeys (MAC-1) was found to be highly related to the MMC-1 cDNA probe, whereas the other retroviral RNAs tested showed no homology. Related sequences were found in Old World monkey DNAs and to a lesser extent in gorilla dn chimpanzee DNAs. No homology was detected between MMC-1 cDNA and DNA of gibbon, orangutan, or human origin. Restriction endonuclease analysis of genomic DNA indicated that many of the several hundred sequences related to MMC-1 in rhesus monkey DNA differed from that integrated into DNA of infected canine cells. Gorilla and chimpanzee DNAs contained a specific restriction endonuclease fragment of the MMC-1 genome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Benton C. V., Hodge H. M., Fine D. L. Comparative large-scale propagation of retroviruses from Old World (Mason-Pfizer monkey virus) and New World (squirrel monkey virus) primates. In Vitro. 1978 Feb;14(2):192–199. doi: 10.1007/BF02618222. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: evidence for an Asian origin of man. Nature. 1976 May 13;261(5556):101–108. doi: 10.1038/261101a0. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Todaro G. J. Carnivores have sequences in their cellular DNA distantly related to the primate endogenous virus, MAC-1. Virology. 1979 Apr 15;94(1):224–227. doi: 10.1016/0042-6822(79)90454-9. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Charman H. P., Gardner M. B., McAllister R. M., Kim N., Gilden R. V. Humoral immune responses of cats to mammalian type-C virus p30s. Int J Cancer. 1976 Jan 15;17(1):98–108. doi: 10.1002/ijc.2910170114. [DOI] [PubMed] [Google Scholar]
- Lovinger G. G., Schochetman G. 5' terminal nucleotide sequences of type C retroviruses: features common to noncoding sequences of eucaryotic messenger RNAs. Cell. 1980 Jun;20(2):441–449. doi: 10.1016/0092-8674(80)90630-3. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Rabin H., Benton C. V., Tainsky M. A., Rice N. R., Gilden R. V. Isolation and characterization of an endogenous type C virus of rhesus monkeys. Science. 1979 May 25;204(4395):841–842. doi: 10.1126/science.87013. [DOI] [PubMed] [Google Scholar]
- Rabin H., Neubauer R. H., Gonda M. A., Nelson-Rees W. A., Charman H. P., Valerio M. G. Spontaneous esophageal carcinoma and epithelial cell line of an adult rhesus monkey. Cancer Res. 1978 Oct;38(10):3310–3314. [PubMed] [Google Scholar]
- Rice N. R., Simek S., Ryder O. A., Coggins L. Detection of proviral DNA in horse cells infected with equine infectious anemia virus. J Virol. 1978 Jun;26(3):577–583. doi: 10.1128/jvi.26.3.577-583.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlom J., Colcher D., Spiegelman S., Gillespie S., Gillespie D. Quantitation of RNA tumor viruses and viruslike particles in human milk by hybridization to polyadenylic acid sequences. Science. 1973 Feb 16;179(4074):696–698. doi: 10.1126/science.179.4074.696. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Sherwin S. A., Sherr C. J. MAC-1, a new genetically transmitted type C virus of primates: "low frequency" activation from stumptail monkey cell cultures. Cell. 1978 Apr;13(4):775–782. doi: 10.1016/0092-8674(78)90227-1. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Benveniste R. E. Baboons and their close relatives are unusual among primates in their ability to release nondefective endogenous type C viruses. Virology. 1976 Jul 1;72(1):278–282. doi: 10.1016/0042-6822(76)90331-7. [DOI] [PubMed] [Google Scholar]