Abstract
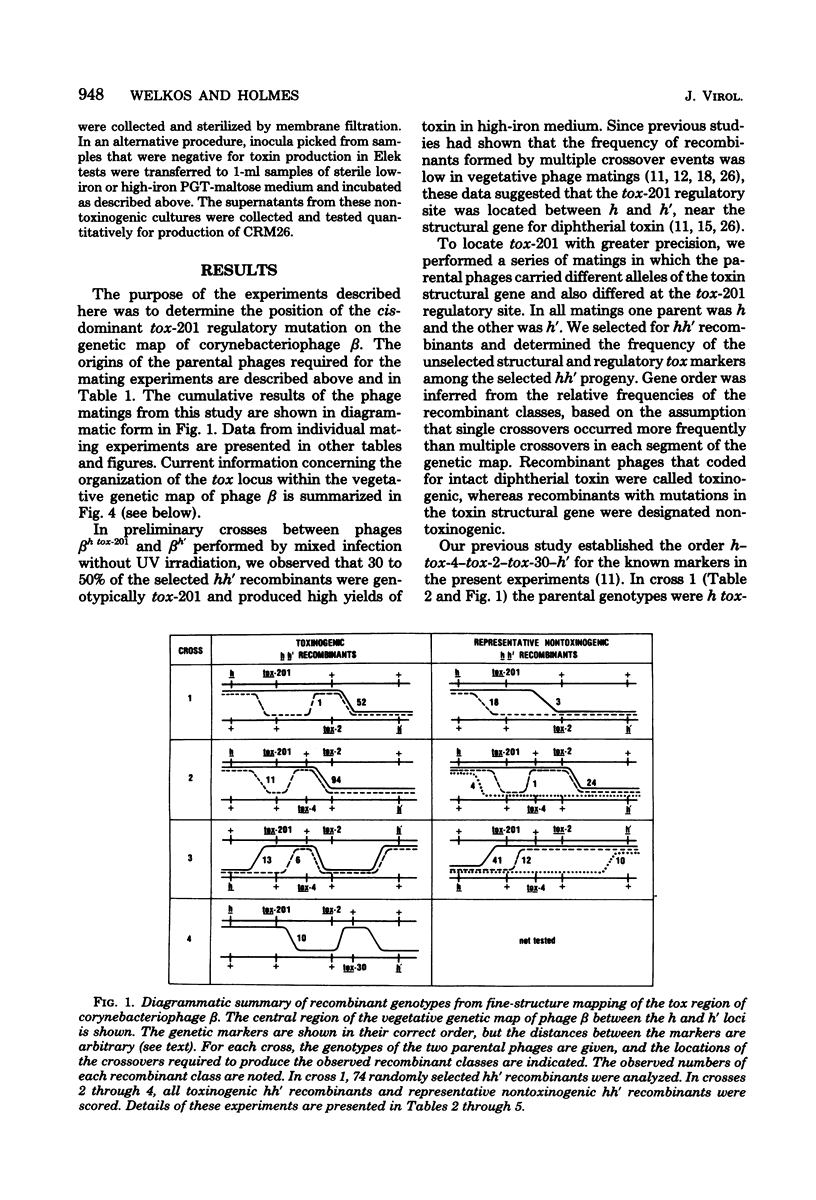
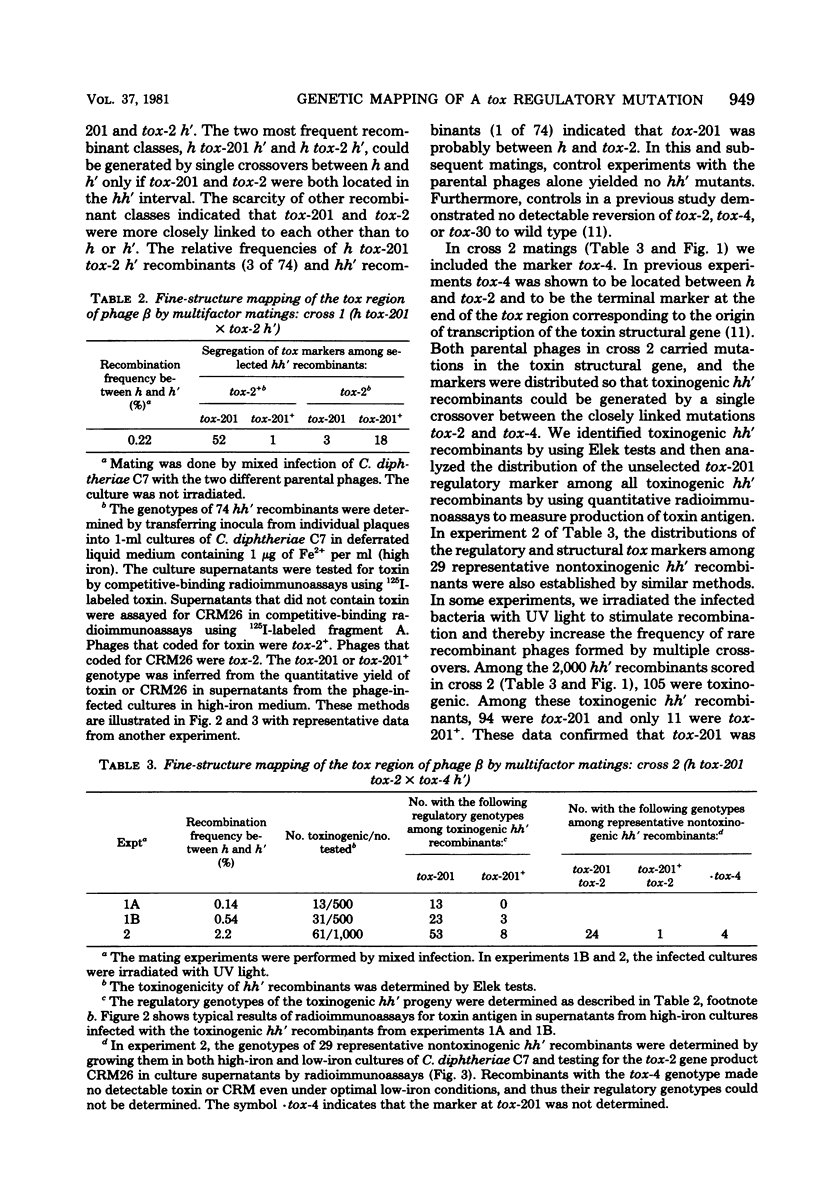
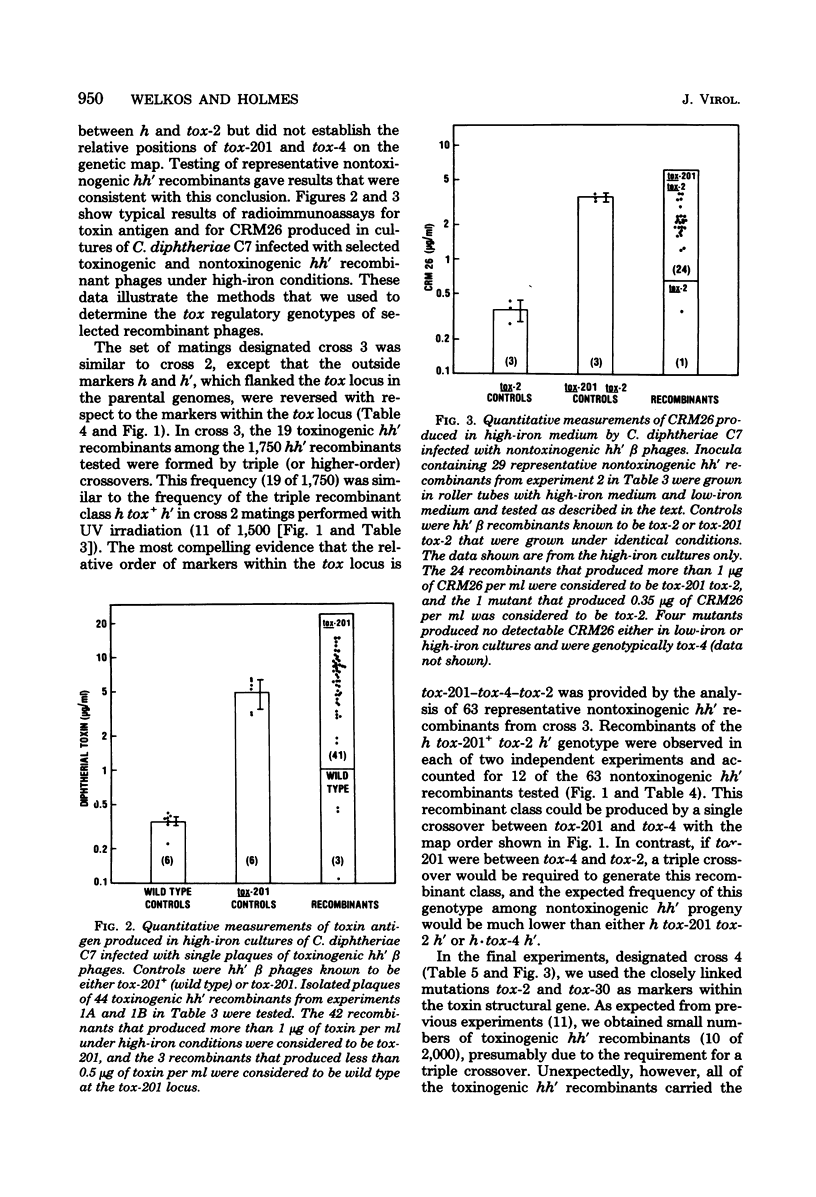
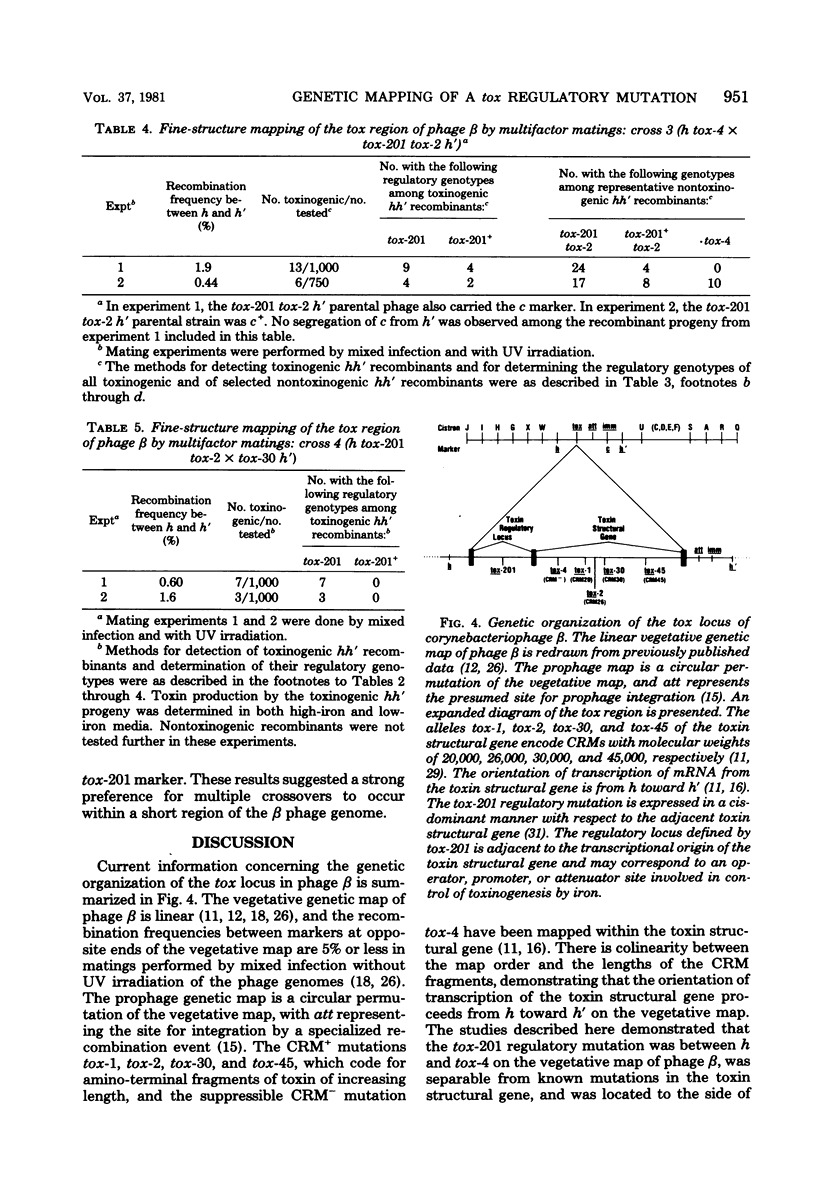
The structural gene for diphtherial toxin is present in corynebacteriophage beta. Previous studies located several point mutations within the tox locus and determined the orientation of transcription of the toxin structural gene. The production of maximal quantities of toxin by Corynebacterium diphtheriae C7(beta) occurs only when the bacteria are iron deficient. Mutations in phage beta can affect this control of toxin production by iron. The tox-201 mutation regulates expression of the toxin structural gene in a cis-dominant manner and permits large amounts of toxin to be made under high-iron conditions when phage beta tox-201 infects C. diphtheriae C7. In this study tox-201 was found to be closely linked to the structural gene for toxin. We performed a series of multifactor matings to determine the relative positions of tox-201 and several point mutations within the toxin structural gene. The order of these markers on the vegetative genetic map of phage beta was tox-201-tox-4-tox-2-tox-30. These findings establish that the tox-201 regulatory site is closely linked to the end of the toxin structural gene corresponding to the origin of transcription. This location is consistent with our hypothesis that tox-201 defines a cis-dominant regulatory element, such as an operator, promoter, or attenuator, involved in control of toxinogenesis in C. diphtheriae C7(beta).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amati P, Meselson M. Localized Negative Interference in Bacteriophage. Genetics. 1965 Mar;51(3):369–379. doi: 10.1093/genetics/51.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacha P., Murphy J. R. Isolation and characterization of extragenic suppressor strains of Corynebacterium diphtheriae. J Bacteriol. 1978 Dec;136(3):1135–1142. doi: 10.1128/jb.136.3.1135-1142.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barksdale L., Arden S. B. Persisting bacteriophage infections, lysogeny, and phage conversions. Annu Rev Microbiol. 1974;28(0):265–299. doi: 10.1146/annurev.mi.28.100174.001405. [DOI] [PubMed] [Google Scholar]
- Chase M, Doermann A H. High Negative Interference over Short Segments of the Genetic Structure of Bacteriophage T4. Genetics. 1958 May;43(3):332–353. doi: 10.1093/genetics/43.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordone L., Radman M. Pyrimidine dimer-induced enhancement of recombination among lambda bacteriophages. Virology. 1970 May;41(1):166–169. doi: 10.1016/0042-6822(70)90064-4. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Welkos S. L., Holmes R. K. Immunochemical studies of diphtherial toxin and related nontoxic mutant proteins. Infect Immun. 1980 Dec;30(3):835–846. doi: 10.1128/iai.30.3.835-846.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. S. Some features of genetic recombination in procaryotes. Annu Rev Genet. 1978;12:47–68. doi: 10.1146/annurev.ge.12.120178.000403. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Uchida T., Singer R. A. Expression of diphtheria toxin genes carried by integrated and nonintegrated phage beta. Virology. 1972 Dec;50(3):664–668. doi: 10.1016/0042-6822(72)90420-5. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D. The production of recombinants in phage crosses. Cold Spring Harb Symp Quant Biol. 1958;23:19–46. doi: 10.1101/sqb.1958.023.01.006. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Barksdale L. Genetic analysis of tox+ and tox- bacteriophages of Corynebacterium diphtheriae. J Virol. 1969 Jun;3(6):586–598. doi: 10.1128/jvi.3.6.586-598.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K. Characterization and genetic mapping of nontoxinogenic (tox) mutants of corynebacteriophage beta. J Virol. 1976 Jul;19(1):195–207. doi: 10.1128/jvi.19.1.195-207.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. L. Etude génétique d'un bactériophage tempéré d'Escherichia coli. III. Effet du rayonnement ultraviolet sur la recombinaison génétique. Ann Inst Pasteur (Paris) 1955 Jun;88(6):724–749. [PubMed] [Google Scholar]
- Kanei C., Uchida T., Yoneda M. Isolation from corynebacterium diphtheriae C7(beta) of bacterial mutants that produce toxin in medium with excess iron. Infect Immun. 1977 Oct;18(1):203–209. doi: 10.1128/iai.18.1.203-209.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird W., Groman N. Orientation of the tox gene in the prophage of corynebacteriophage beta. J Virol. 1976 Jul;19(1):228–231. doi: 10.1128/jvi.19.1.228-231.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird W., Groman N. Prophage map of converting corynebacteriophage beta. J Virol. 1976 Jul;19(1):208–219. doi: 10.1128/jvi.19.1.208-219.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone R. E., Chattoraj D. K., Faulds D. H., Stahl M. M., Stahl F. W. Hotspots for generalized recombination in the Escherichia coli chromosome. J Mol Biol. 1978 Jun 5;121(4):473–491. doi: 10.1016/0022-2836(78)90395-9. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Kanei C., Yoneda M. Temperature-sensitive mutants on nonlysogenizing corynebacteriophage hv : their isolation, characterization and relation to toxiongenesis. Biken J. 1971 Jun;14(2):119–129. [PubMed] [Google Scholar]
- Murphy J. R., Michel J. L., Teng M. Evidence that the regulation of diphtheria toxin production is directed at the level of transcription. J Bacteriol. 1978 Aug;135(2):511–516. doi: 10.1128/jb.135.2.511-516.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Pappenheimer A. M., Jr, de Borms S. T. Synthesis of diphtheria tox-gene products in Escherichia coli extracts. Proc Natl Acad Sci U S A. 1974 Jan;71(1):11–15. doi: 10.1073/pnas.71.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Skiver J., McBride G. Isolation and partial characterization of a corynebacteriophage beta, tox operator constitutive-like mutant lysogen of Corynebacterium diphtheriae. J Virol. 1976 Apr;18(1):235–244. doi: 10.1128/jvi.18.1.235-244.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. Marker-specific effects in genetic recombination. J Mol Biol. 1970 Aug;51(3):633–655. doi: 10.1016/0022-2836(70)90013-6. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Sprague K. U., Faulds D. H., Smith G. R. A single base-pair change creates a Chi recombinational hotspot in bacteriophage lambda. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6182–6186. doi: 10.1073/pnas.75.12.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Greany R. Diphtheria toxin and related proteins. I. Isolation and properties of mutant proteins serologically related to diphtheria toxin. J Biol Chem. 1973 Jun 10;248(11):3838–3844. [PubMed] [Google Scholar]
- Welkos S. L., Holmes R. K. Characterization of a screening test for diphtherial toxin antigen produced by individual plaques of corynebacteriophages. J Clin Microbiol. 1979 Jun;9(6):693–698. doi: 10.1128/jcm.9.6.693-698.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welkos S. L., Holmes R. K. Regulation of toxinogenesis in Corynebacterium diphtheriae. I. Mutations in bacteriophage beta that alter the effects of iron on toxin production. J Virol. 1981 Mar;37(3):936–945. doi: 10.1128/jvi.37.3.936-945.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. L., Fox M. S. On the molecular basis of high negative interference. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1544–1548. doi: 10.1073/pnas.71.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R., Fox M. S. Genetic heterozygosity in unreplicated bacteriophage lambda recombinants. Genetics. 1975 Sep;81(1):33–50. doi: 10.1093/genetics/81.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]


