Abstract
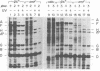
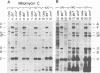
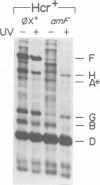
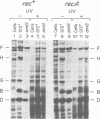
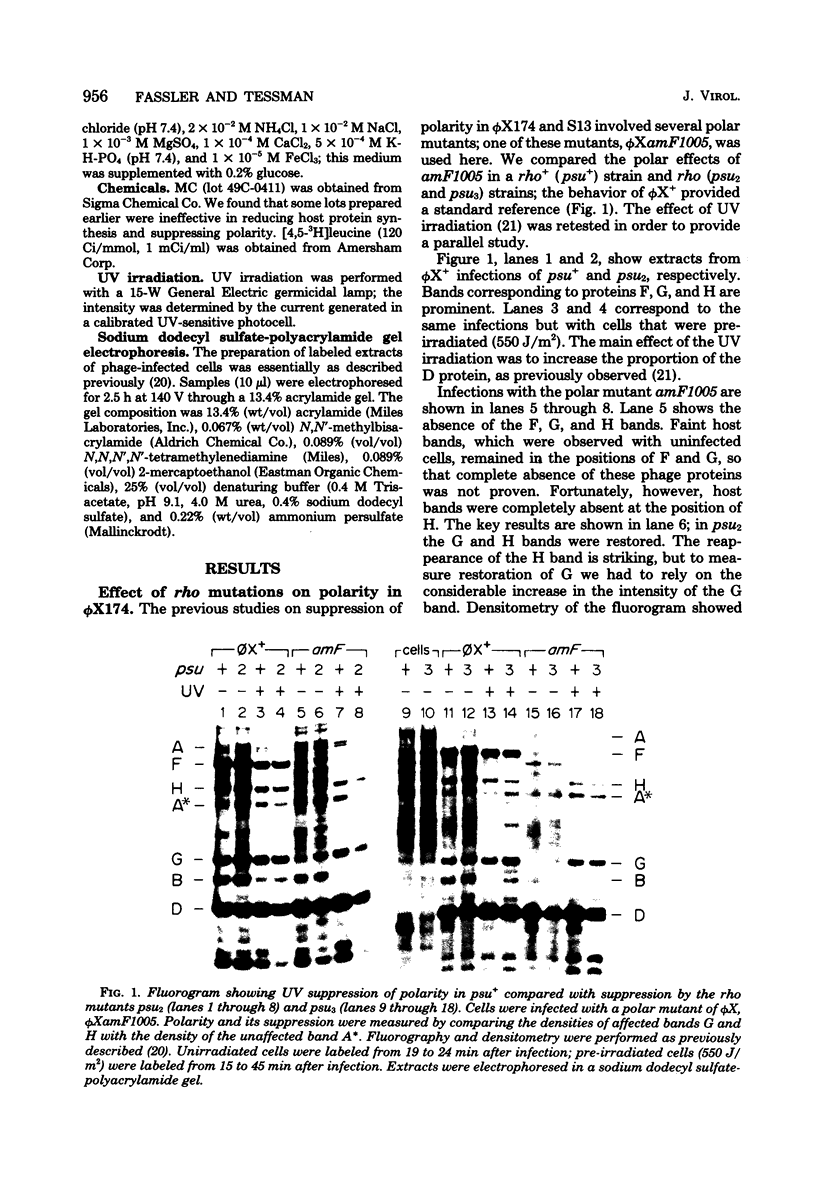
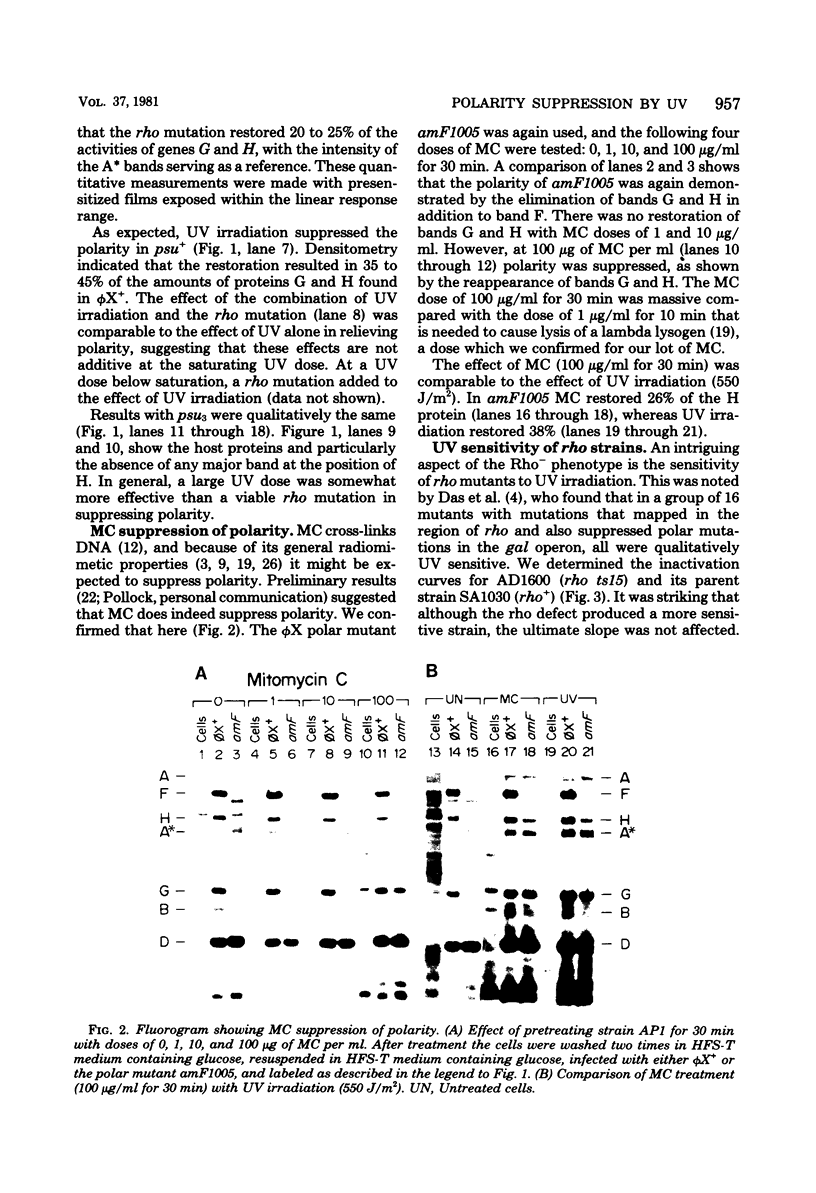
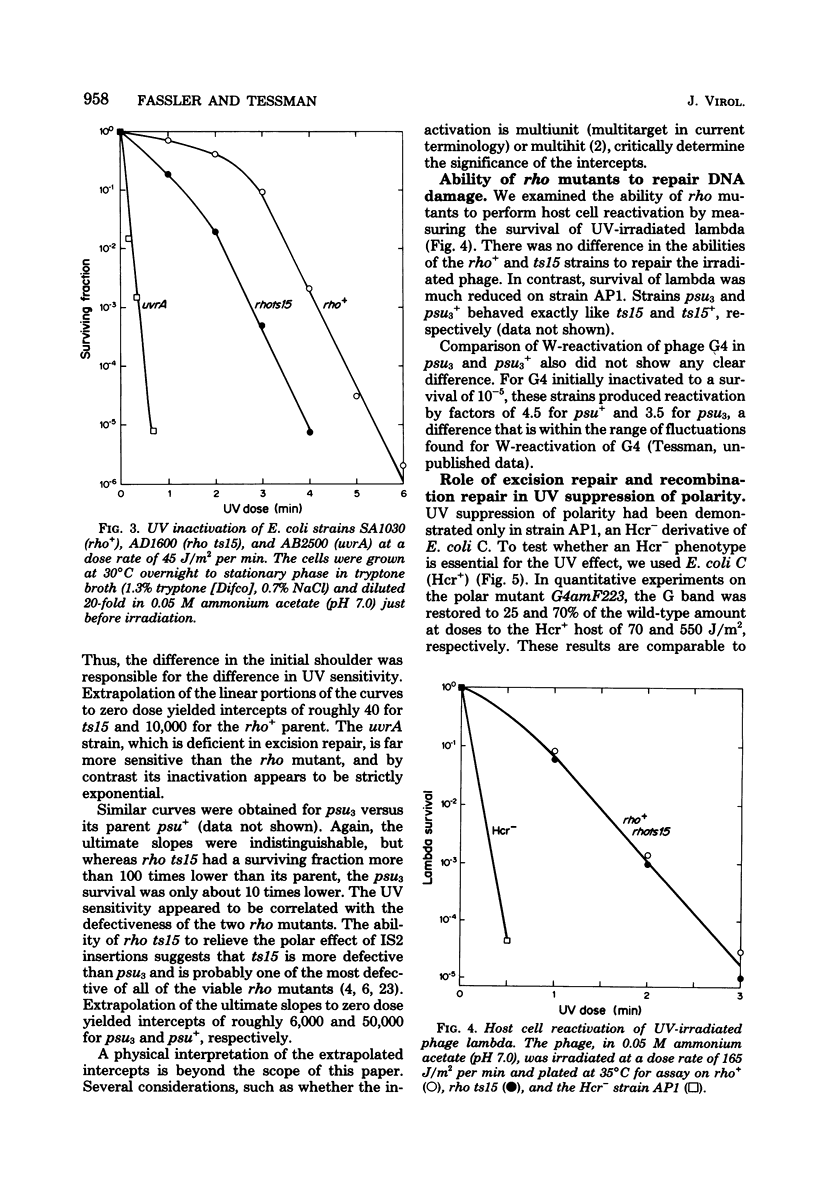
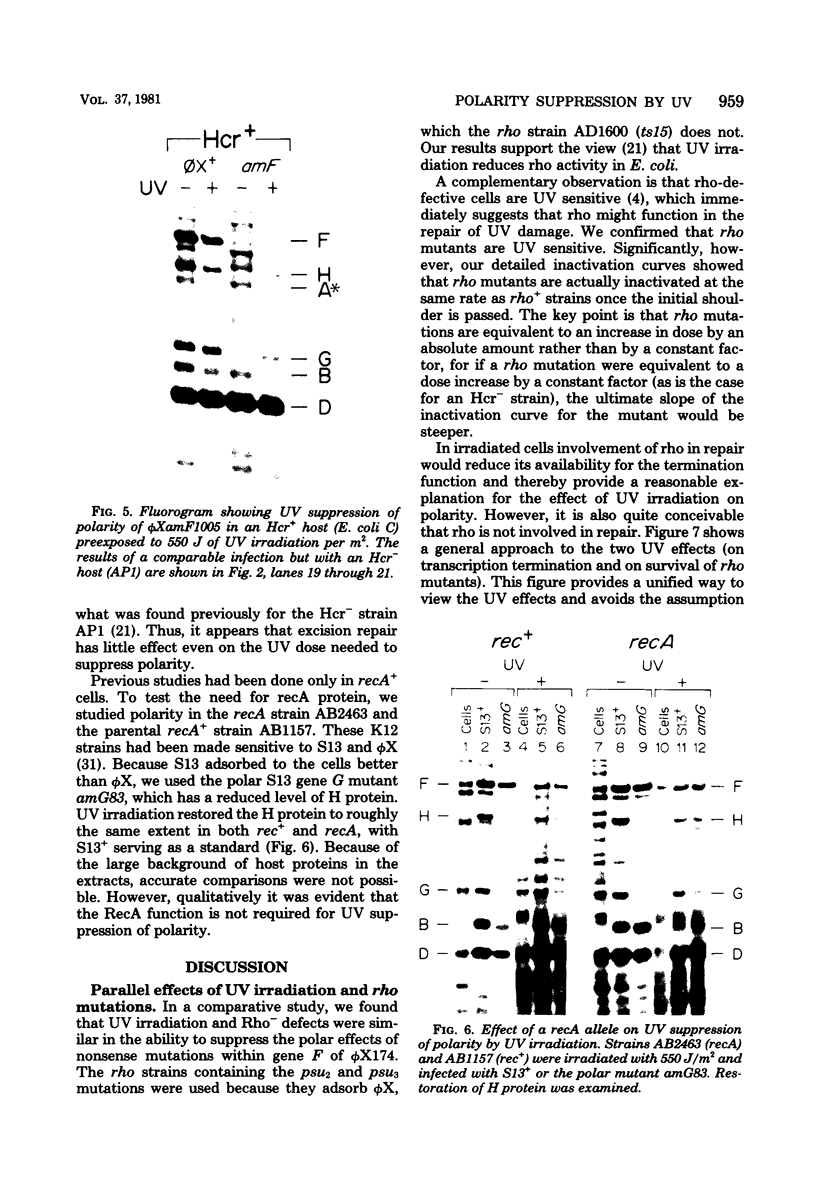
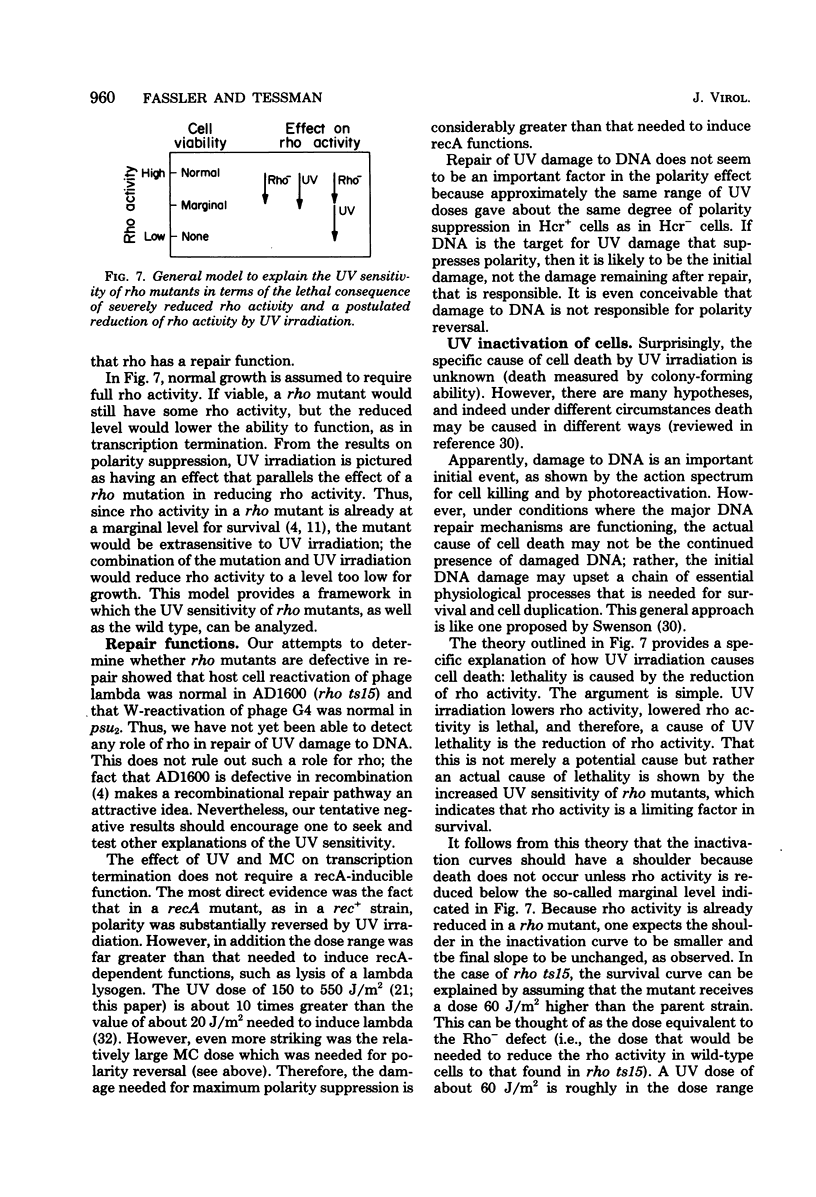
The suppression of polarity by UV irradiation was similar to the suppression by rho mutants. This was demonstrated for a polar nonsense mutant of phage phi X174. Treatment of the host for 30 min with 100 micrograms of the radiomimetic drug mitomycin C per ml was about as effective as 550 J of UV irradiation per m2 in relieving polarity. The shape of the UV survival curves for rho mutants could be linked to a proposed mechanism of UV relief of polarity. Host cell reactivation of phage lambda and W-reactivation of phage G4 were unaffected by rho mutations. UV suppression of polarity is independent of the Hcr- and RecA- phenotypes. An explanation for the UV sensitivity of rho mutants is provided, and several ways are considered in which UV irradiation may deplete cellular rho activity and thereby cause UV relief of polarity. We propose a novel theory that relates the UV inactivation of normal repair-proficient cells to a decrease in rho activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Atwood K. C., Norman A. On the Interpretation of Multi-Hit Survival Curves. Proc Natl Acad Sci U S A. 1949 Dec;35(12):696–709. doi: 10.1073/pnas.35.12.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. GENETIC CONTROL OF DNA BREAKDOWN AND REPAIR IN E. COLI K-12 TREATED WITH MITOMYCIN C OR ULTRAVIOLET LIGHT. Z Vererbungsl. 1964 Dec 30;95:345–350. doi: 10.1007/BF01268667. [DOI] [PubMed] [Google Scholar]
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Adhya S., Gottesman M., Pastan I. Effect of Rho on transcription of bacterial operons. Nat New Biol. 1973 Feb 28;241(113):260–264. doi: 10.1038/newbio241260a0. [DOI] [PubMed] [Google Scholar]
- Howard B. H., de Crombrugghe B. ATPase activity required for termination of transcription by the Escherichia coli protein factor rho. J Biol Chem. 1976 Apr 25;251(8):2520–2524. [PubMed] [Google Scholar]
- IIJIMA T., HAGIWARA A. Mutagenic action of mitomycin C on Escherichia coli. Nature. 1960 Feb 6;185:395–396. doi: 10.1038/185395b0. [DOI] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. A MOLECULAR MECHANISM OF MITOMYCIN ACTION: LINKING OF COMPLEMENTARY DNA STRANDS. Proc Natl Acad Sci U S A. 1963 Aug;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto F. Evidence for premature termination of transcription of the tryptophan operon in polarity mutants of Escherichia coli. Nature. 1970 Oct 17;228(5268):232–235. doi: 10.1038/228232a0. [DOI] [PubMed] [Google Scholar]
- Inoko H., Shigesada K., Imai M. Isolation and characterization of conditional-lethal rho mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1162–1166. doi: 10.1073/pnas.74.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan E., Saedler H., Lengeler J., Starlinger P. Changes in the specific activities of the galactose enzymes in E. coli under different growth conditions. Mol Gen Genet. 1967;100(2):203–209. doi: 10.1007/BF00333606. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Yanofsky C. Polarity suppressors defective in transcription termination at the attenuator of the tryptophan operon of Escherichia coli have altered rho factor. J Mol Biol. 1976 Sep 15;106(2):231–241. doi: 10.1016/0022-2836(76)90082-6. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Yanofsky C. Polarity suppressors increase expression of the wild-type tryptophan operon of Escherichia coli. J Mol Biol. 1976 May 15;103(2):395–409. doi: 10.1016/0022-2836(76)90319-3. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. Process of infection with bacteriophage phi-X174. XIV. Studies on macromolecular synthesis during infection with a lysis-defective mutant. J Mol Biol. 1967 Aug 28;28(1):87–94. doi: 10.1016/s0022-2836(67)80079-2. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Primakoff P. Relief of polarity in E. coli by "suA". Nature. 1970 Apr 4;226(5240):28–31. doi: 10.1038/226028a0. [DOI] [PubMed] [Google Scholar]
- OTSUJI N., SEKIGUCHI M., IIJIMA T., TAKAGI Y. Induction of phage formation in the lysogenic Escherichia coli K-12 by mitomycin C. Nature. 1959 Oct 3;184(Suppl 14):1079–1080. doi: 10.1038/1841079b0. [DOI] [PubMed] [Google Scholar]
- Pollock T. J. Gene D of bacteriophage phi X 174: absence of transcriptional and translational regulatory properties. J Virol. 1977 Feb;21(2):468–474. doi: 10.1128/jvi.21.2.468-474.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock T. J., Tessman E. S., Tessman I. Suppression of polar effects of nonsense mutations by ultraviolet irradiation. J Bacteriol. 1979 Apr;138(1):122–125. doi: 10.1128/jb.138.1.122-125.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner D. Evidence that mutations in the suA polarity suppressing gene directly affect termination factor rho. Nature. 1976 Jan 15;259(5539):151–153. doi: 10.1038/259151a0. [DOI] [PubMed] [Google Scholar]
- Reyes O., Gottesman M., Adhya S. Suppression of polarity of insertion mutations in the gal operon and N mutations in bacteriophage lambda. J Bacteriol. 1976 Jun;126(3):1108–1112. doi: 10.1128/jb.126.3.1108-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P., Grimley C., Lowery C. Transcription termination factor rho activity is altered in Escherichia coli with suA gene mutations. Proc Natl Acad Sci U S A. 1975 May;72(5):1725–1728. doi: 10.1073/pnas.72.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A., Adhya S. L. The galactose operon of E. coli K-12. II. A deletion analysis of operon structure and polarity. Genetics. 1969 Jun;62(2):249–264. doi: 10.1093/genetics/62.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. Mutations caused by the insertion of genetic material into the galactose operon of Escherichia coli. J Mol Biol. 1969 Feb 28;40(1):93–105. doi: 10.1016/0022-2836(69)90298-8. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Gottesman M., Tomczak K., Gottesman S. Hyperdegradation of proteins in Escherichia coli rho mutants. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1623–1627. doi: 10.1073/pnas.76.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman I. Genetic recombination of phage S13 in a recombination-deficient mutant of Escherichia coli K12. Biochem Biophys Res Commun. 1966 Jan 24;22(2):169–174. doi: 10.1016/0006-291x(66)90427-x. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa T. Effect of ultraviolet irradiation on bacteriophage lambda immunity. J Mol Biol. 1967 Jan 28;23(2):247–263. doi: 10.1016/s0022-2836(67)80031-7. [DOI] [PubMed] [Google Scholar]