Abstract
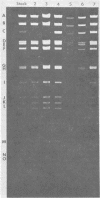
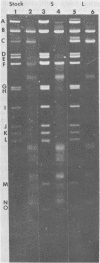
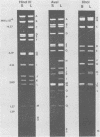
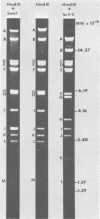
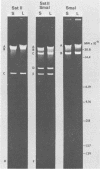
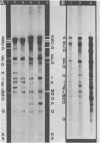
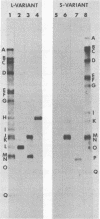
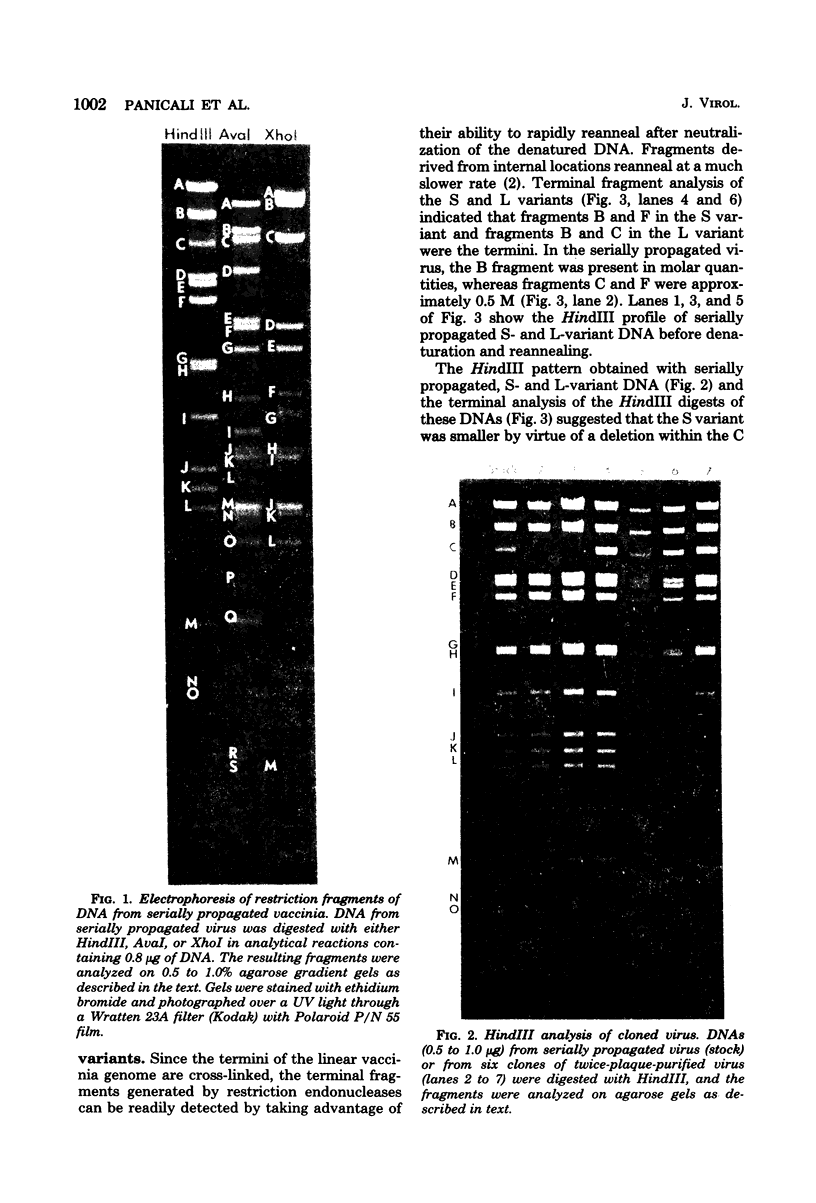
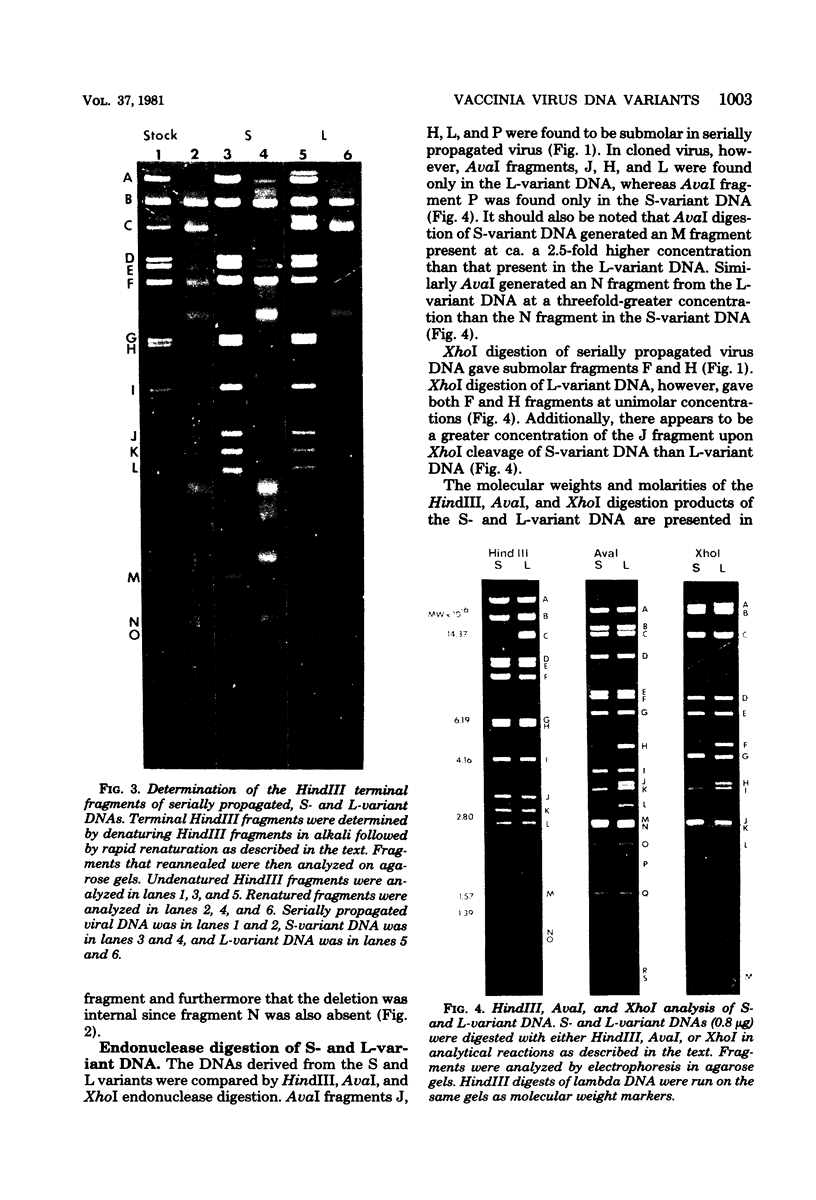
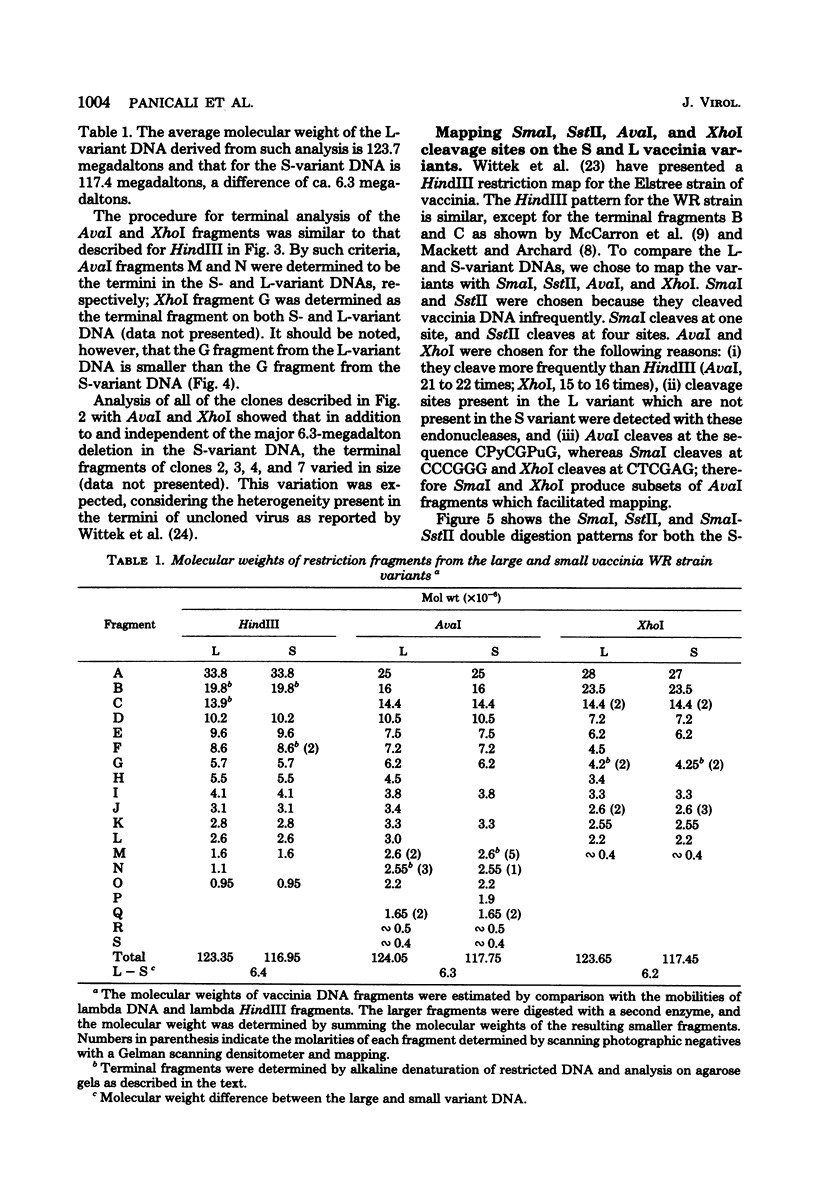
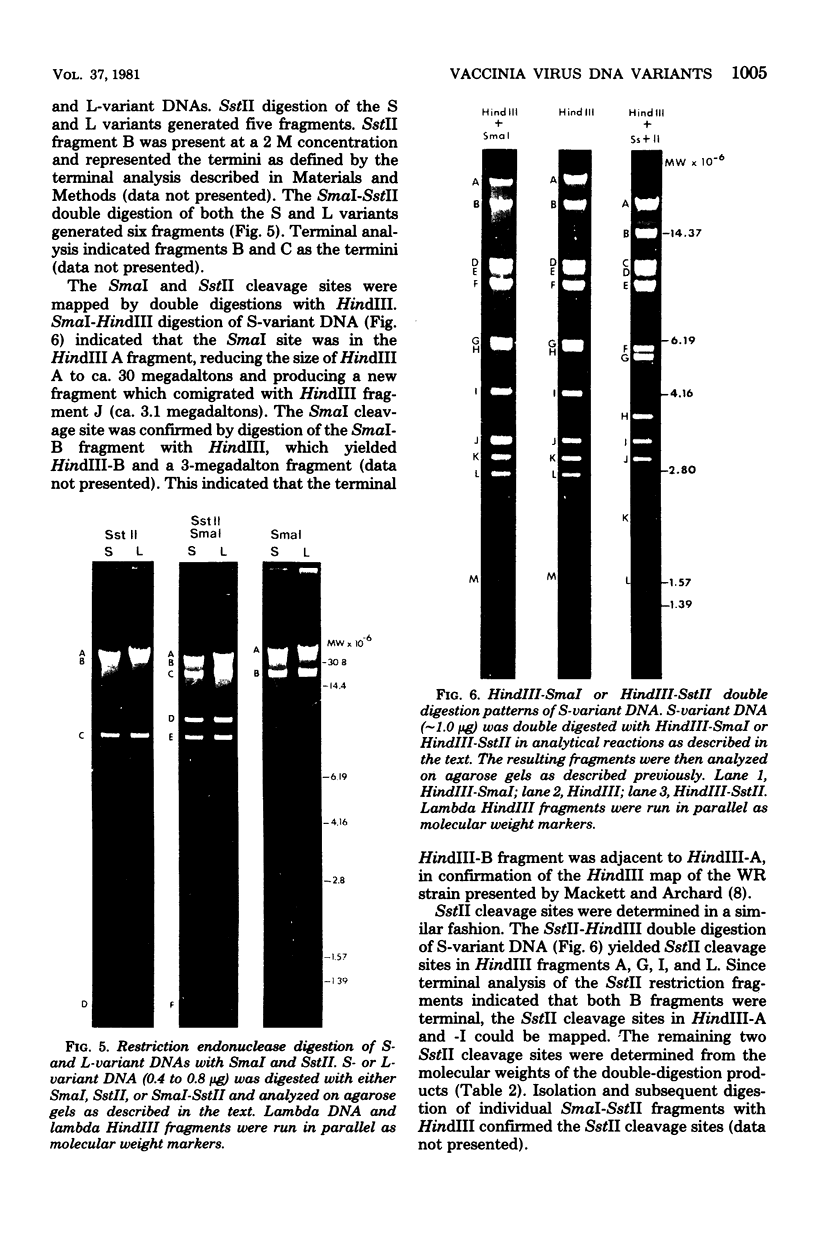
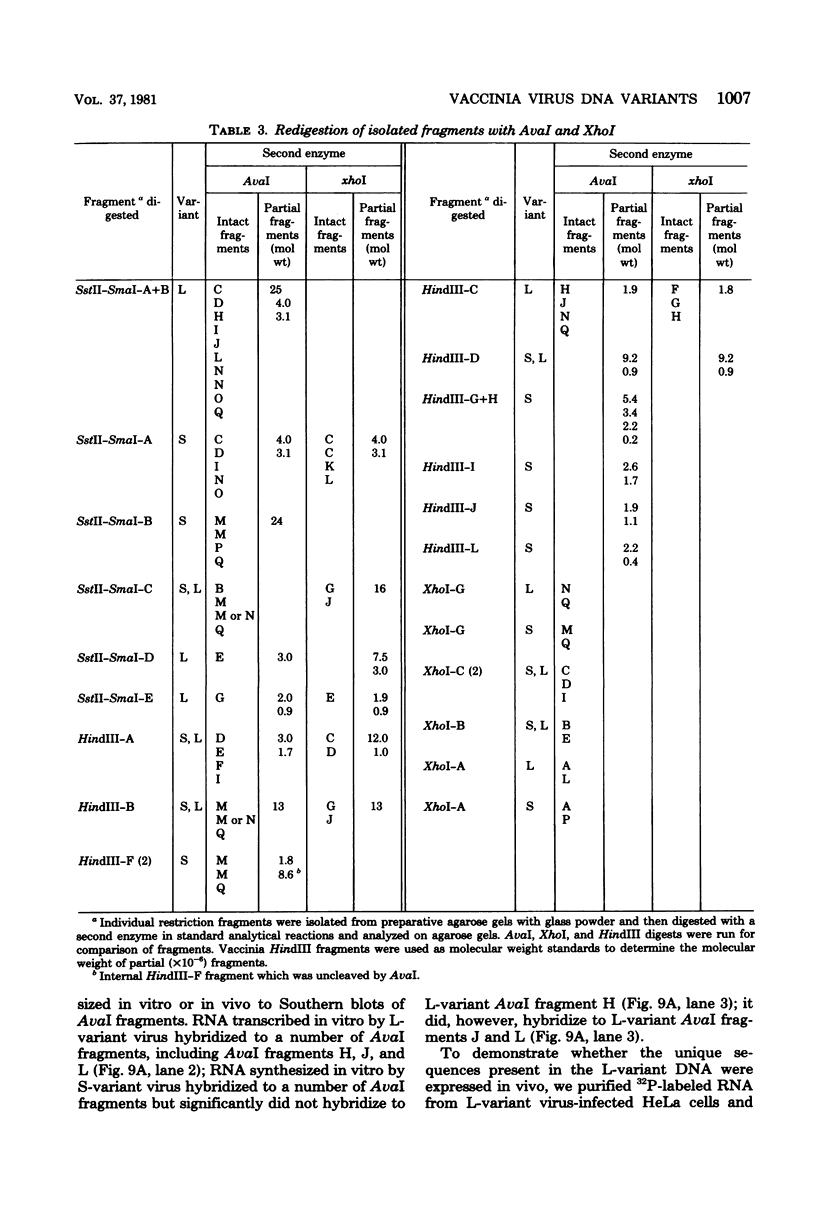
Two major DNA variants have been isolated from serially propagated stocks of the WR strain of vaccinia virus. Restriction enzyme mapping of the two variants with HindIII, AvaI, XhoI, SstII, and SmaI revealed a 6.3-megadalton deletion in the smaller DNA variant. The deletion was mapped at ca. 6.8 megadaltons in from the left terminus, just beyond the inverted terminal repeat. The additional DNA present in the larger variant was found to represent unique viral sequences that were transcribed both in vitro and in vivo. One-step growth curves in HeLa cells revealed no difference in rat of replication or burst size when progeny was scored on CV-1 monolayers.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archard L. C., Mackett M. Restriction endonuclease analysis of red cowpox virus and its white pock variant. J Gen Virol. 1979 Oct;45(1):51–63. doi: 10.1099/0022-1317-45-1-51. [DOI] [PubMed] [Google Scholar]
- DeFilippes F. Restriction enzyme digests of rapidly renaturing fragments of vaccinia virus DNA. J Virol. 1975 Jan;17(1):227–238. doi: 10.1128/jvi.17.1.227-238.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangemi J. D., Sharp D. G. Use of a restriction endonuclease in analyzing the genomes from two different strains of vaccinia virus. J Virol. 1976 Oct;20(1):319–323. doi: 10.1128/jvi.20.1.319-323.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon C. F., Barbosa E., Moss B. Visualization of an inverted terminal repetition in vaccinia virus DNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4863–4867. doi: 10.1073/pnas.75.10.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geshelin P., Berns K. I. Characterization and localization of the naturally occurring cross-links in vaccinia virus DNA. J Mol Biol. 1974 Oct 5;88(4):785–796. doi: 10.1016/0022-2836(74)90399-4. [DOI] [PubMed] [Google Scholar]
- Grady L. J., Paoletti E. Molecular complexity of vaccinia DNA and the presence of reiterated sequences in the genome. Virology. 1977 Jun 15;79(2):337–341. doi: 10.1016/0042-6822(77)90361-0. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Mackett M., Archard L. C. Conservation and variation in Orthopoxvirus genome structure. J Gen Virol. 1979 Dec;45(3):683–701. doi: 10.1099/0022-1317-45-3-683. [DOI] [PubMed] [Google Scholar]
- McCarron R. J., Cabrera C. V., Esteban M., McAllister W. T., Holowczak J. A. Structure of vaccinia DNA: analysis of the viral genome by restriction endonucleases. Virology. 1978 May 1;86(1):88–101. doi: 10.1016/0042-6822(78)90010-7. [DOI] [PubMed] [Google Scholar]
- McFadden G., Dales S. Biogenesis of poxviruses: mirror-image deletions in vaccinia virus DNA. Cell. 1979 Sep;18(1):101–108. doi: 10.1016/0092-8674(79)90358-1. [DOI] [PubMed] [Google Scholar]
- Mears J. G., Ramirez F., Leibowitz D., Nakamura F., Bloom A., Konotey-Ahulu F., Bank A. Changes in restricted human cellular DNA fragments containing globin gene sequences in thalassemias and related disorders. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1222–1226. doi: 10.1073/pnas.75.3.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer R. W., Rothe C. T. The white pock mutants of rabbit poxvirus. I. Spontaneous host range mutants contain deletions. Virology. 1980 Apr 15;102(1):119–132. doi: 10.1016/0042-6822(80)90075-6. [DOI] [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R., Panicali D. Capped and polyadenylated low-molecular-weight RNA synthesized by vaccinia virus in vitro. J Virol. 1980 Jan;33(1):208–219. doi: 10.1128/jvi.33.1.208-219.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrali-Noy G., Weissbach A. Evidence of a repetitive sequence in vaccinia virus DNA. J Virol. 1977 Oct;24(1):406–407. doi: 10.1128/jvi.24.1.406-407.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schümperli D., Menna A., Schwendimann F., Wittek R., Wyler R. Symmetrical arrangement of the heterologous regions of rabbit poxvirus and vaccinia virus DNA. J Gen Virol. 1980 Apr;47(2):385–398. doi: 10.1099/0022-1317-47-2-385. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Menna A., Müller H. K., Schümperli D., Boseley P. G., Wyler R. Inverted terminal repeats in rabbit poxvirus and vaccinia virus DNA. J Virol. 1978 Oct;28(1):171–181. doi: 10.1128/jvi.28.1.171-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Menna A., Schümperli D., Stoffel S., Müller H. K., Wyler R. HindIII and Sst I restriction sites mapped on rabbit poxvirus and vaccinia virus DNA. J Virol. 1977 Sep;23(3):669–678. doi: 10.1128/jvi.23.3.669-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Müller H. K., Wyler R. Length heterogeneity in the DNA of vaccinia virus is eliminated on cloning the virus. FEBS Lett. 1978 Jun 1;90(1):41–46. doi: 10.1016/0014-5793(78)80293-2. [DOI] [PubMed] [Google Scholar]