Abstract
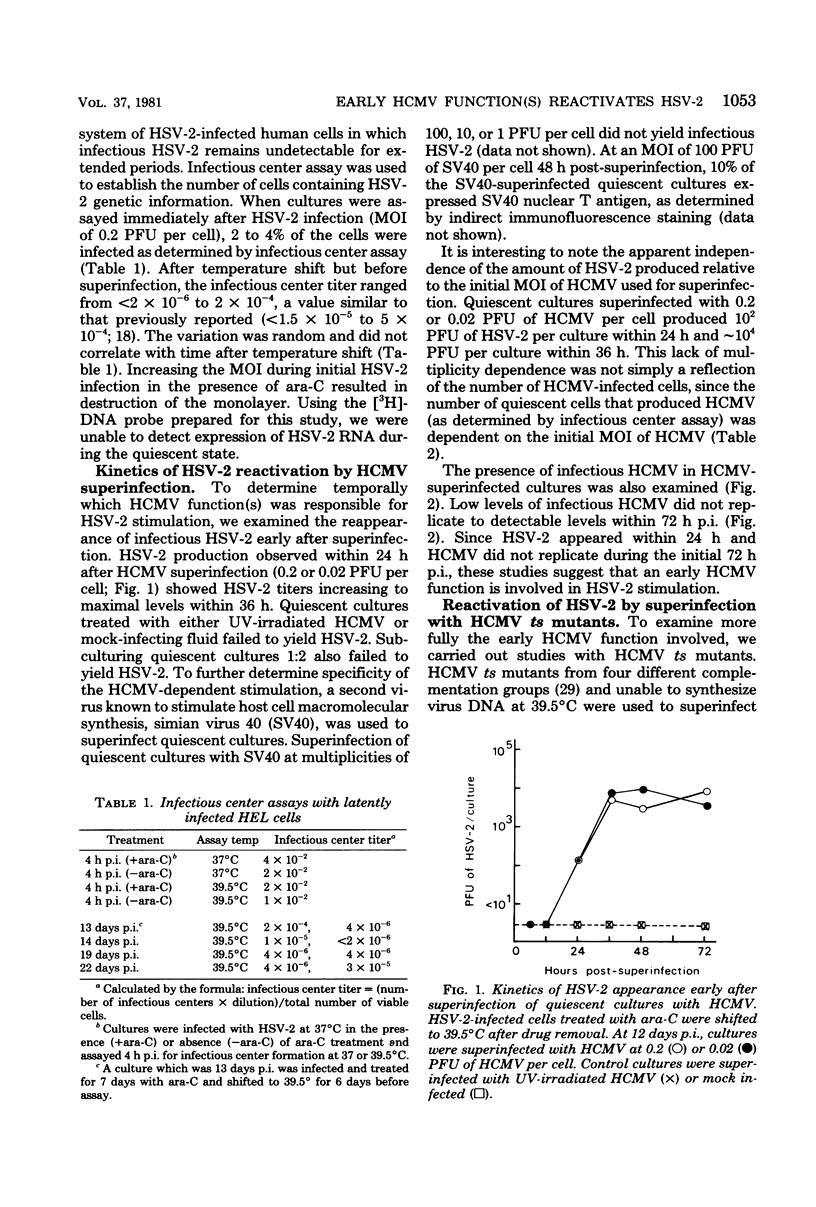
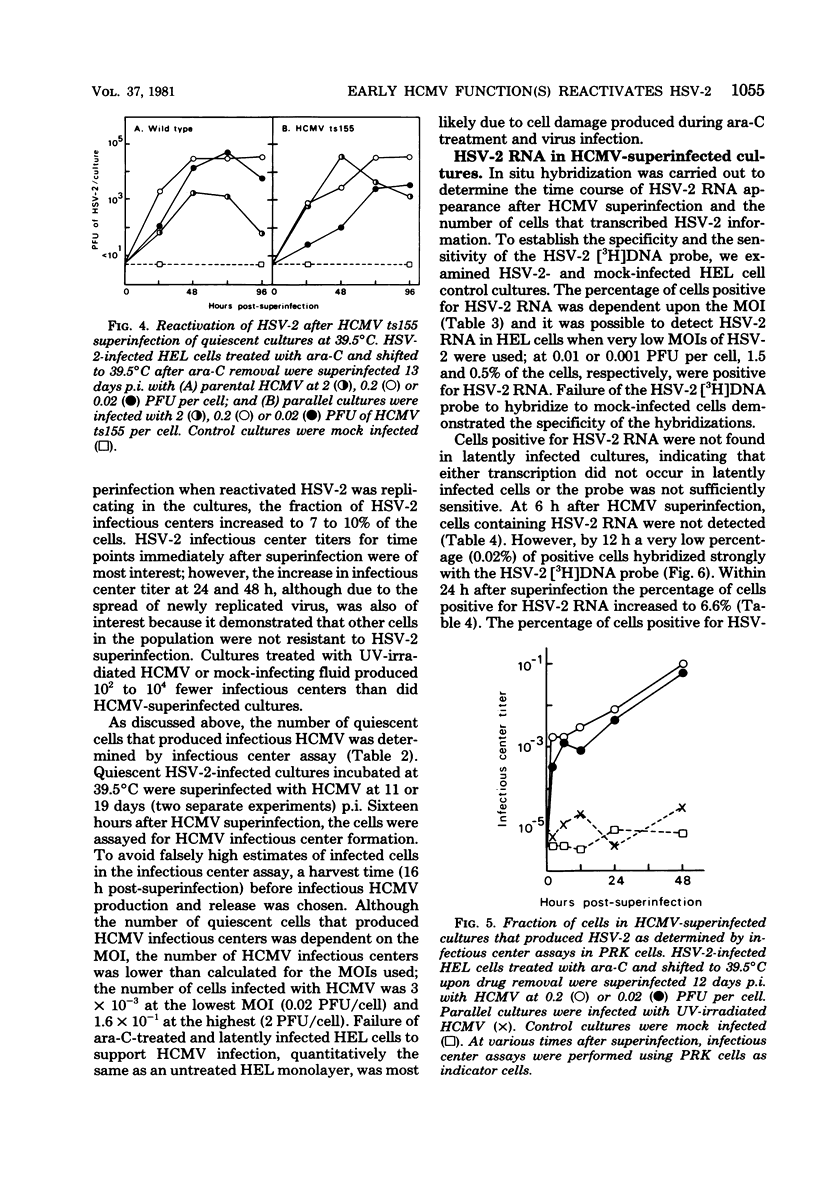
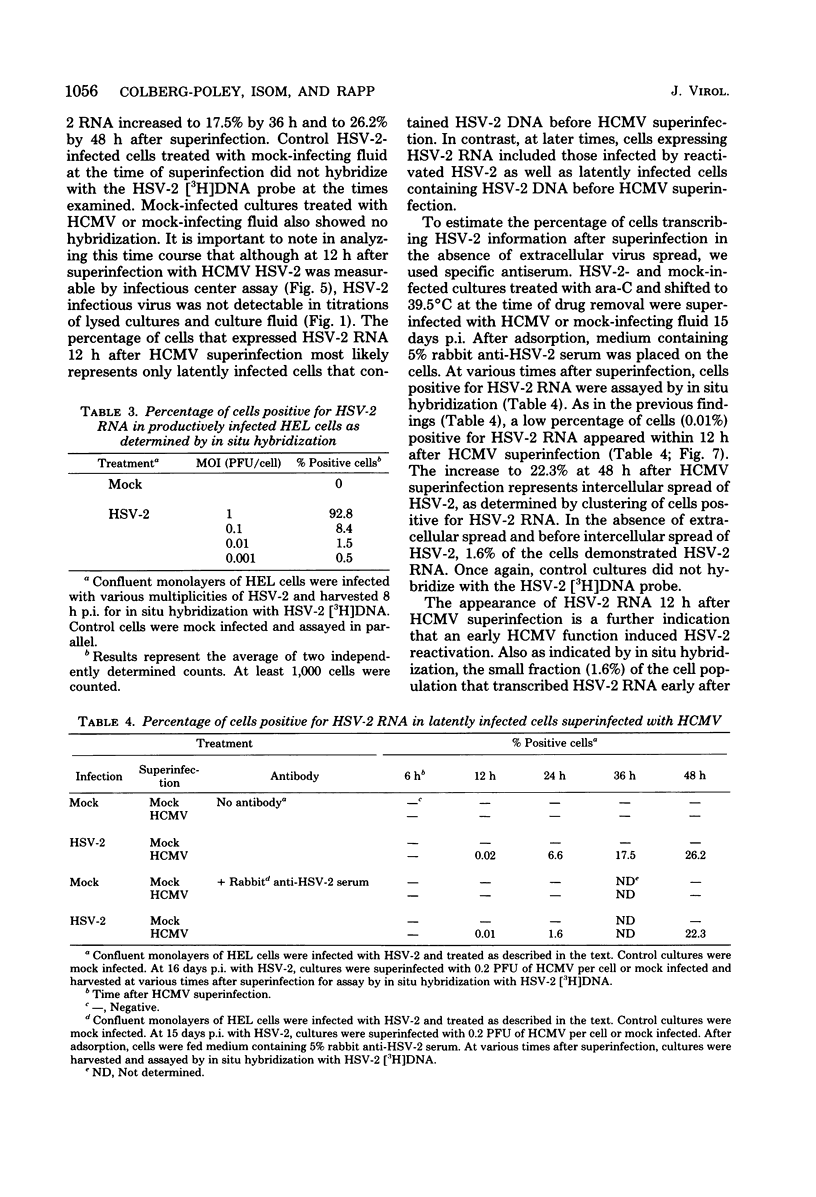
We have previously described an in vitro system in which the function lacking for herpes simplex virus type 2 (HSV-2) replication can be induced by human cytomegalovirus (HCMV). The mechanism of this reactivation of quiescent HSV-2 by HCMV has been further defined. The HCMV function(s) responsible for HSV-2 stimulation was examined temporally, and the fraction of cells in quiescent cultures producing HSV-2 after superinfection was determined. Using independent biological, genetic and molecular techniques we have made the following observations. (i) As early as 12 h after HCMV superinfection, HSV-2 RNA was expressed in latently infected cells. (ii) At 24 h after HCMV superinfection, a time when newly synthesized HCMV was not yet apparent, infectious HSV-2 was produced by reactivated cultures. (iii) Four HCMV temperature-sensitive mutants, which are DNA-negative at nonpermissive temperature and represent four different complementation groups, induced reactivation of HSV-2 at 39.5 degrees C. (iv) Early after HCMV superinfection, 1.6% of quiescent cells could be induced to transcribe HSV-2 information. (v) Early after HCMV superinfection, 0.3% of cells in the quiescent cultures could be induced to yield infectious HSV-2. The finding that a significant interaction can occur between HCMV and quiescent HSV-2 in an in vitro model is noteworthy in light of the knowledge that both of these herpesviruses often reside simultaneously in the human host.
Full text
PDF

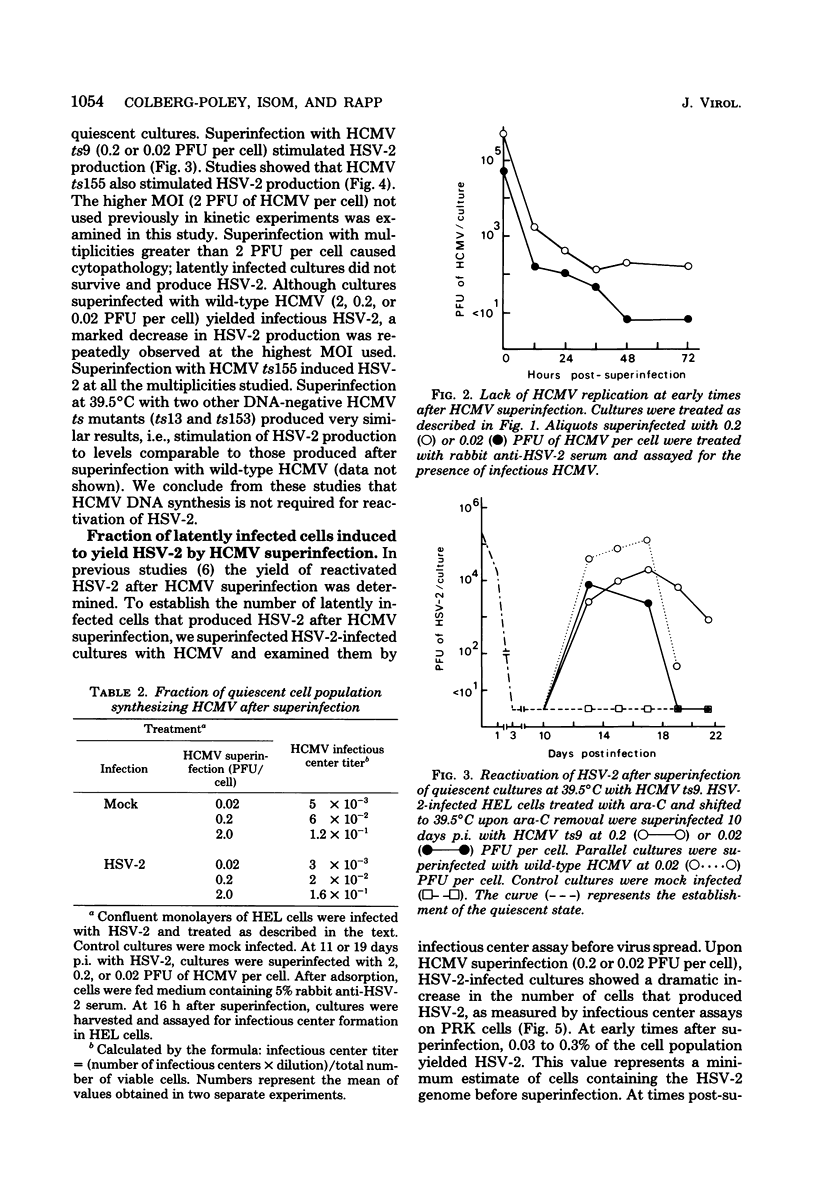



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baringer J. R. Recovery of herpes simplex virus from human sacral ganglions. N Engl J Med. 1974 Oct 17;291(16):828–830. doi: 10.1056/NEJM197410172911606. [DOI] [PubMed] [Google Scholar]
- Baringer J. R., Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973 Mar 29;288(13):648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- Bastian F. O., Rabson A. S., Yee C. L., Tralka T. S. Herpesvirus hominis: isolation from human trigeminal ganglion. Science. 1972 Oct 20;178(4058):306–307. doi: 10.1126/science.178.4058.306. [DOI] [PubMed] [Google Scholar]
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Isom H. C., Rapp F. Reactivation of herpes simplex virus type 2 from a quiescent state by human cytomegalovirus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5948–5951. doi: 10.1073/pnas.76.11.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Isom H., Rapp F. Experimental HSV latency using phosphonoacetic acid. Proc Soc Exp Biol Med. 1979 Oct;162(1):235–237. doi: 10.3181/00379727-162-40655. [DOI] [PubMed] [Google Scholar]
- Copple C. D., McDougall J. K. Clonal derivatives of a herpes type 2 transformed hamster cell line (333-8-9): cytogenetic analysis, tumorigenicity and virus sequence detection. Int J Cancer. 1976 Apr 15;17(4):501–510. doi: 10.1002/ijc.2910170413. [DOI] [PubMed] [Google Scholar]
- Docherty J. J., Mitchell W. R., Thompson C. J., Anthony A. Abortive herpes simplex virus replication in Rous sarcoma virus transformed cells. Proc Soc Exp Biol Med. 1973 Nov;144(2):697–704. doi: 10.3181/00379727-144-37665. [DOI] [PubMed] [Google Scholar]
- Doller E., Aucker J., Weissbach A. Persistence of herpes simplex virus type 1 in rat neurotumor cells. J Virol. 1979 Jan;29(1):43–50. doi: 10.1128/jvi.29.1.43-50.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIL FERNANDEZ C. Persistence of herpes simplex virus in HeLa cells. Nature. 1960 Jan 23;185:268–268. doi: 10.1038/185268a0. [DOI] [PubMed] [Google Scholar]
- Galloway D. A., Fenoglio C., Shevchuk M., McDougall J. K. Detection of herpes simplex RNA in human sensory ganglia. Virology. 1979 May;95(1):265–268. doi: 10.1016/0042-6822(79)90429-x. [DOI] [PubMed] [Google Scholar]
- Gerdes J. C., Marsden H. S., Cook M. L., Stevens J. G. Acute infection of differentiated neuroblastoma cells by latency-positive and latency-negative herpes simplex virus ts mutants. Virology. 1979 Apr 30;94(2):430–441. doi: 10.1016/0042-6822(79)90473-2. [DOI] [PubMed] [Google Scholar]
- Hampar B., Copeland M. L. Persistent Herpes Simplex Virus Infection In Vitro with Cycles of Cell Destruction and Regrowth. J Bacteriol. 1965 Jul;90(1):205–212. doi: 10.1128/jb.90.1.205-212.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Pagano J. S. Human cytomegalovirus. II. Lack of relatedness to DNA of herpes simples I and II, Epstein-Barr virus, and nonhuman strains of cytomegalovirus. J Virol. 1974 Mar;13(3):642–645. doi: 10.1128/jvi.13.3.642-645.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty K. S., Jr, Vollmer R. T., McCarty K. S. Improved computer program data for the resolution and fractionation of macromolecules by isokinetic sucrose density gradient sedimentation. Anal Biochem. 1974 Sep;61(1):165–183. doi: 10.1016/0003-2697(74)90343-1. [DOI] [PubMed] [Google Scholar]
- Michelson S., Horodniceanu F., Kress M., Tardy-Panit M. Human cytomegalovirus-induced immediate early antigens: analysis in sodium dodecyl sulfate-polyacrylamide gel electrophoresis after immunoprecipitation. J Virol. 1979 Oct;32(1):259–267. doi: 10.1128/jvi.32.1.259-267.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill F. J., Goldberg R. J., Rapp F. Herpes simplex virus latency in cultured human cells following treatment with cytosine arabinoside. J Gen Virol. 1972 Feb;14(2):189–197. doi: 10.1099/0022-1317-14-2-189. [DOI] [PubMed] [Google Scholar]
- O'Neill F. J. Prolongation of herpes simplex virus latency in cultured human cells by temperature elevation. J Virol. 1977 Oct;24(1):41–46. doi: 10.1128/jvi.24.1.41-46.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda S., Jack I., White D. O. Herpes-simplex virus from trigeminal ganglion. Lancet. 1973 Jun 16;1(7816):1395–1396. doi: 10.1016/s0140-6736(73)91730-3. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Nesburn A. B., Cook M. L. Latent herpes simplex virus from trigeminal ganglia of rabbits with recurrent eye infection. Nat New Biol. 1972 Feb 16;235(59):216–217. doi: 10.1038/newbio235216a0. [DOI] [PubMed] [Google Scholar]
- Stinski M. F. Sequence of protein synthesis in cells infected by human cytomegalovirus: early and late virus-induced polypeptides. J Virol. 1978 Jun;26(3):686–701. doi: 10.1128/jvi.26.3.686-701.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F. Synthesis of proteins and glycoproteins in cells infected with human cytomegalovirus. J Virol. 1977 Sep;23(3):751–767. doi: 10.1128/jvi.23.3.751-767.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHEELER C. E. The effect of temperature upon the production of herpes simplex virus in tissue culture. J Immunol. 1958 Aug;81(2):98–106. [PubMed] [Google Scholar]
- Warren K. G., Brown S. M., Wroblewska Z., Gilden D., Koprowski H., Subak-Sharpe J. Isolation of latent herpes simplex virus from the superior cervical and vagus ganglions of human beings. N Engl J Med. 1978 May 11;298(19):1068–1069. doi: 10.1056/NEJM197805112981907. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Rapp F. Induction of host DNA synthesis and DNA polymerase by DNA-negative temperature-sensitive mutants of human cytomegalovirus. Virology. 1979 Apr 15;94(1):237–241. doi: 10.1016/0042-6822(79)90457-4. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Rapp F. Temperature-sensitive mutants of human cytomegalovirus. J Virol. 1977 Oct;24(1):416–418. doi: 10.1128/jvi.24.1.416-418.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]