Abstract
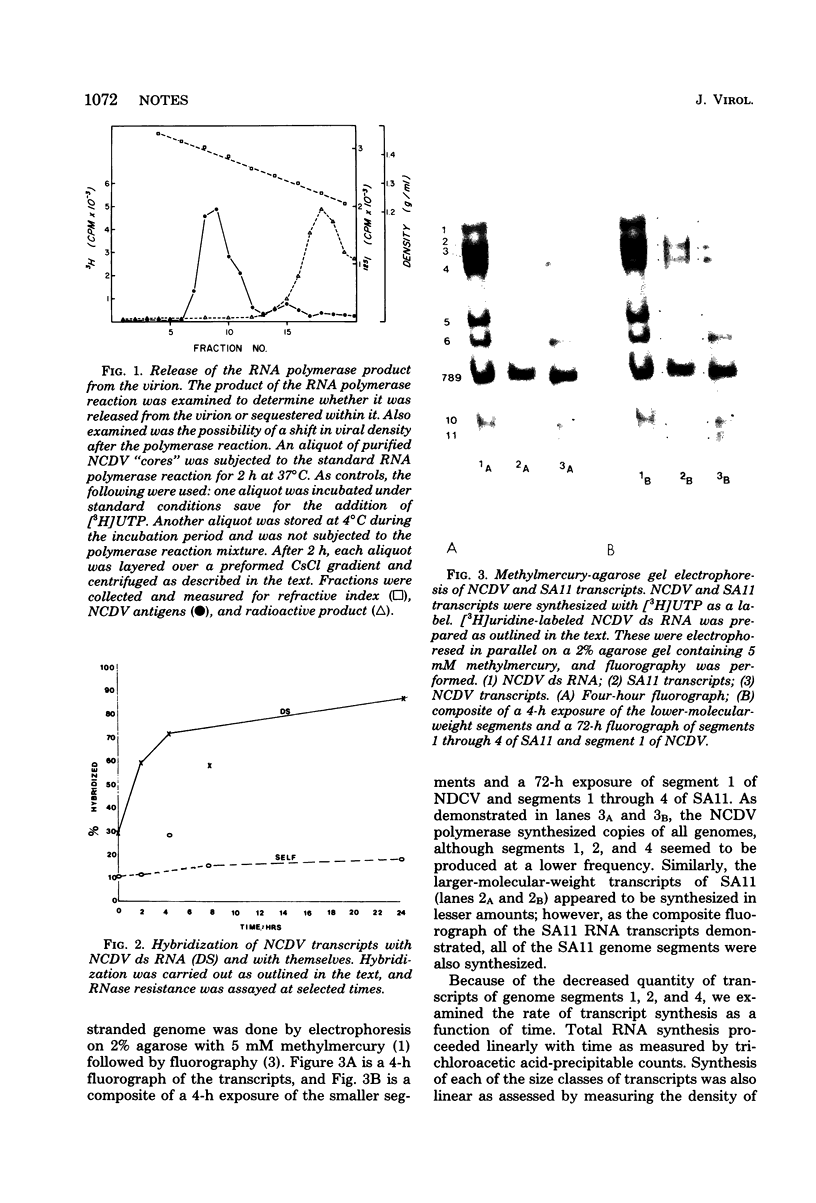
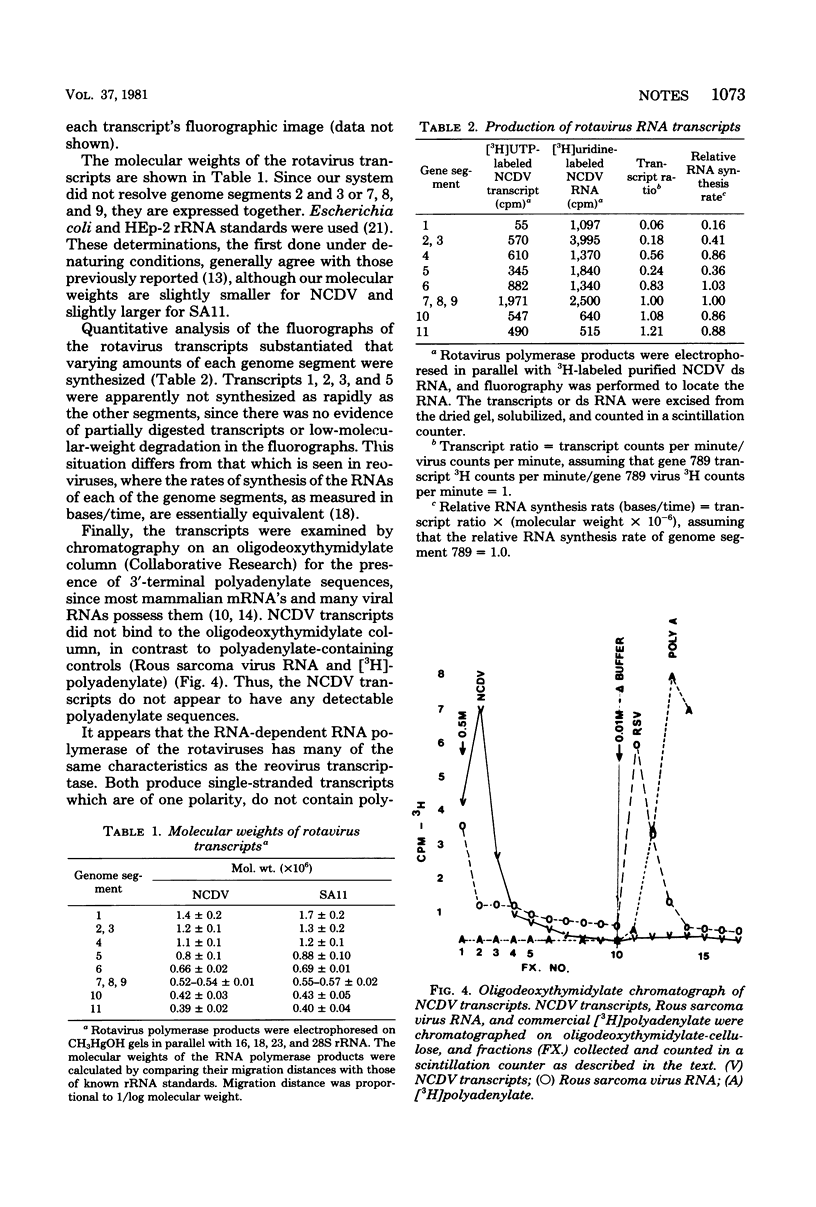
The endogenous RNA polymerase of the calf rotavirus was shown to synthesize single-stranded RNA transcripts of one polarity which were identical in size to the denatured parental double-stranded RNA segments. The transcripts were notable in their absence of polyadenylate sequences. The polymerase activity associated with the cores of calf rotavirus was minimally altered by the reaction, since calf rotavirus cores could be reused after purification, whereas the RNA polymerase products appeared to be released from the virion.
Full text
PDF

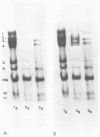
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Purification and properties of poliovirus double-stranded ribonucleic acid. J Mol Biol. 1966 Jul;18(3):421–428. doi: 10.1016/s0022-2836(66)80034-7. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bridger J. C. Location of type-specific antigens in calf rotaviruses. J Clin Microbiol. 1978 Dec;8(6):625–628. doi: 10.1128/jcm.8.6.625-628.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Dobos P. Cell free transcription and translation of rotavirus RNA. Biochem Biophys Res Commun. 1979 Jun 13;88(3):791–796. doi: 10.1016/0006-291x(79)91477-3. [DOI] [PubMed] [Google Scholar]
- Cohen J., Laporte J., Charpilienne A., Scherrer R. Activation of rotavirus RNA polymerase by calcium chelation. Arch Virol. 1979;60(3-4):177–186. doi: 10.1007/BF01317489. [DOI] [PubMed] [Google Scholar]
- Cohen J. Ribonucleic acid polymerase activity associated with purified calf rotavirus. J Gen Virol. 1977 Sep;36(3):395–402. doi: 10.1099/0022-1317-36-3-395. [DOI] [PubMed] [Google Scholar]
- Flewett T. H., Woode G. N. The rotaviruses. Arch Virol. 1978;57(1):1–23. doi: 10.1007/BF01315633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J., Joklik W. K. Demonstration that the same strand of reovirus genome RNA is transcribed in vitro and in vivo. Virology. 1971 May;44(2):450–453. doi: 10.1016/0042-6822(71)90276-5. [DOI] [PubMed] [Google Scholar]
- Hruska J. F., Notter M. F., Menegus M. A., Steinhoff M. C. RNA polymerase associated with human rotaviruses in diarrhea stools. J Virol. 1978 May;26(2):544–546. doi: 10.1128/jvi.26.2.544-546.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Sereno M. M., Wyatt R. G., Mebus C. A., Chanock R. M., Kapikian A. Z. Comparison of human and animal rotavirus strains by gel electrophoresis of viral RNA. Virology. 1978 Jun 15;87(2):247–255. doi: 10.1016/0042-6822(78)90130-7. [DOI] [PubMed] [Google Scholar]
- Karpetsky T. P., Boguski M. S., Levy C. C. Structures, properties, and possible biologic functions of polyadenylic acid. Subcell Biochem. 1979;6:1–116. doi: 10.1007/978-1-4615-7945-8_1. [DOI] [PubMed] [Google Scholar]
- MALHERBE H., HARWIN R. The cytopathic effects of vervet monkey viruses. S Afr Med J. 1963 Apr 20;37:407–411. [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. In vitro transcription and translation of simian rotavirus SA11 gene products. J Virol. 1980 Mar;33(3):1111–1121. doi: 10.1128/jvi.33.3.1111-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Christman J. K., Acs G. The reovirus replicative cycle. Annu Rev Biochem. 1976;45:375–408. doi: 10.1146/annurev.bi.45.070176.002111. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Joklik W. K. Studies on the in vitro transcription of reovirus RNA catalyzed by reovirus cores. Virology. 1969 Dec;39(4):822–831. doi: 10.1016/0042-6822(69)90019-1. [DOI] [PubMed] [Google Scholar]
- Smith M. L., Lazdins I., Holmes I. H. Coding assignments of double-stranded RNA segments of SA 11 rotavirus established by in vitro translation. J Virol. 1980 Mar;33(3):976–982. doi: 10.1128/jvi.33.3.976-982.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Shatkin A. J., Banerjee A. K. Absence of polyadenylic acid from reovirus messenger ribonucleic acid. J Biol Chem. 1973 Dec 10;248(23):7993–7998. [PubMed] [Google Scholar]