Abstract
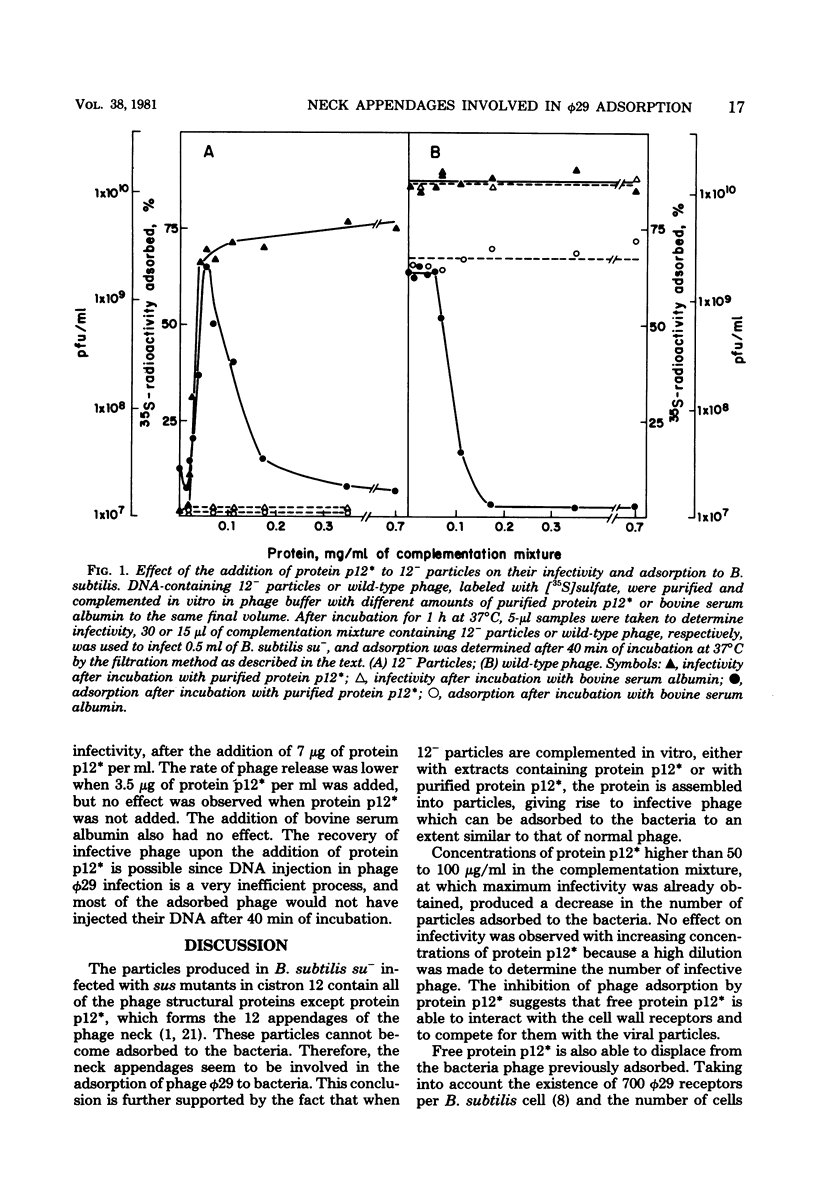
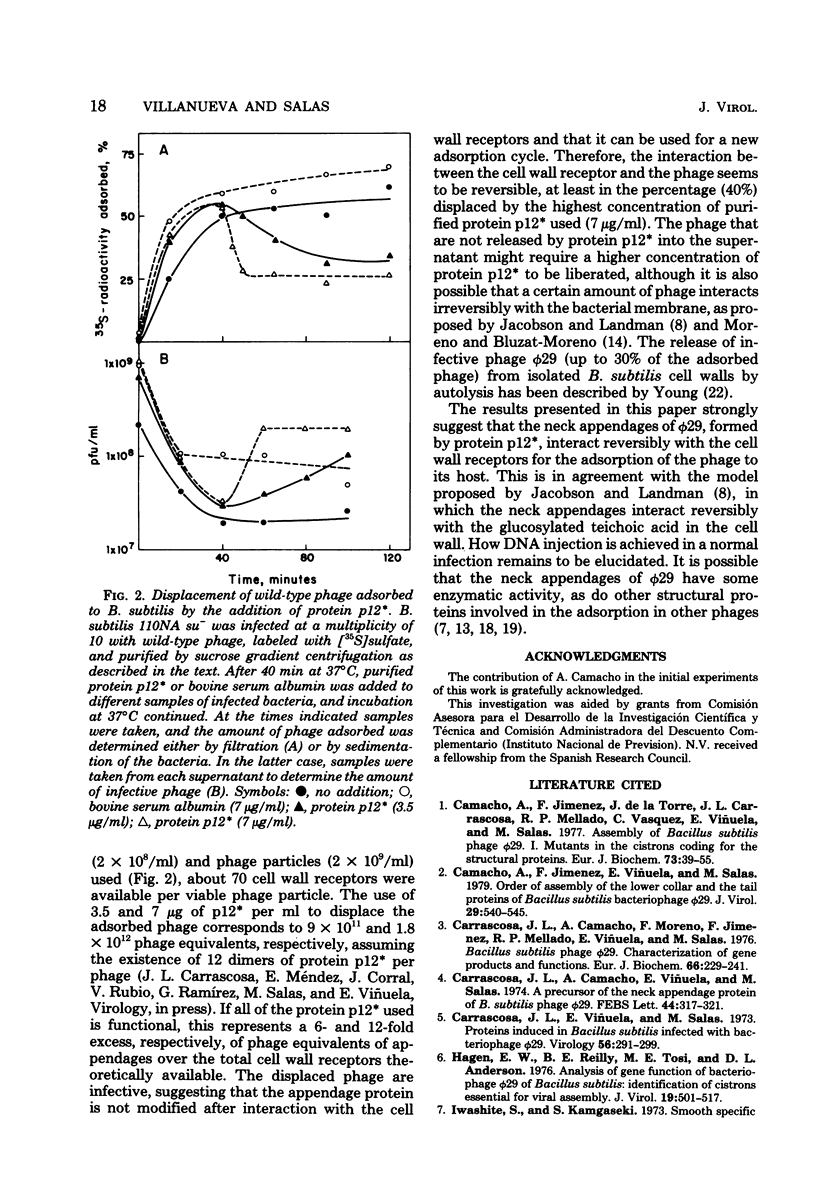
Phage phi 29 particles produced under restrictive conditions by mutants in gene 12 have normal amounts of all of the structural proteins except the appendage protein, p12*, which is missing. These particles are not infective and do not adsorb to Bacillus subtilis cells. By in vitro complementation of 12- particles with extracts containing protein p12* or with purified protein p12*, the defective particles could bind the appendage protein and become infective and able to adsorb to bacteria. Therefore, the neck appendages of phage phi 29, formed by protein p12*, are involved in the interaction of the phage with the cell wall receptors. Protein p12*, purified in its native state, competed with wild-type phage for adsorption to bacteria. Also, protein p12* could displace adsorbed phage from bacteria. Since the displaced phage was infective, protein p12* does not seem to be modified after phage adsorption.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camacho A., Jiménez F., De La Torre J., Carrascosa J. L., Mellado R. P., Vásquez C., Viñuela E., Salas M. Assembly of Bacillus subtilis phage phi29. 1. Mutants in the cistrons coding for the structural proteins. Eur J Biochem. 1977 Feb 15;73(1):39–55. doi: 10.1111/j.1432-1033.1977.tb11290.x. [DOI] [PubMed] [Google Scholar]
- Camacho A., Jiménez F., Viñuela E., Salas M. Order of assembly of the lower collar and the tail proteins of Bacillus subtilis bacteriophage phi 29. J Virol. 1979 Feb;29(2):540–545. doi: 10.1128/jvi.29.2.540-545.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrascosa J. L., Camacho A., Moreno F., Jiménez F., Mellado R. P., Viñuela E., Salas M. Bacillus subtilis phage phi29. Characterization of gene products and functions. Eur J Biochem. 1976 Jul 1;66(2):229–241. doi: 10.1111/j.1432-1033.1976.tb10512.x. [DOI] [PubMed] [Google Scholar]
- Carrascosa J. L., Camacho A., Viñuela E., Salas M. A precursor of the neck appendage protein of B. subtilis phage phi 29. FEBS Lett. 1974 Aug 30;44(3):317–321. [PubMed] [Google Scholar]
- Carrascosa J. L., Viñuela E., Salas M. Proteins induced in Bacillus subtilis infected with bacteriophage phi 29. Virology. 1973 Nov;56(1):291–299. [PubMed] [Google Scholar]
- Hagen E. W., Reilly B. E., Tosi M. E., Anderson D. L. Analysis of gene function of bacteriophage phi 29 of Bacillus subtilis: identification of cistrons essential for viral assembly. J Virol. 1976 Aug;19(2):501–517. doi: 10.1128/jvi.19.2.501-517.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita S., Kanegasaki S. Smooth specific phage adsorption: endorhamnosidase activity of tail parts of P22. Biochem Biophys Res Commun. 1973 Nov 16;55(2):403–409. doi: 10.1016/0006-291x(73)91101-7. [DOI] [PubMed] [Google Scholar]
- Jacobson E. D., Landman O. E. Adsorption of bacteriophages phi 29 and 22a to protoplasts of Bacillus subtilis 168. J Virol. 1977 Mar;21(3):1223–1227. doi: 10.1128/jvi.21.3.1223-1227.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez F., Camacho A., De La Torre J., Viñuela E., Salas M. Assembly of Bacillus subtilis phage phe29. 2. Mutants in the cistrons coding for the non-structural proteins. Eur J Biochem. 1977 Feb 15;73(1):57–72. doi: 10.1111/j.1432-1033.1977.tb11291.x. [DOI] [PubMed] [Google Scholar]
- Mellado R. P., Moreno F., Viñuela E., Salas M., Reilly B. E., Anderson D. L. Genetic analysis of bacteriophage phi 29 of Bacillus subtilis: integration and mapping of reference mutants of two collections. J Virol. 1976 Aug;19(2):495–500. doi: 10.1128/jvi.19.2.495-500.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado R. P., Vinuela E., Salas M. Isolation of a strong suppressor of nonsense mutations in Bacillus subtilis. Eur J Biochem. 1976 May 17;65(1):213–223. doi: 10.1111/j.1432-1033.1976.tb10408.x. [DOI] [PubMed] [Google Scholar]
- Mindich L., Lehman J. Cell wall lysin as a component of the bacteriophage phi 6 virion. J Virol. 1979 May;30(2):489–496. doi: 10.1128/jvi.30.2.489-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno F., Bluzat-Moreno F. G. Evidence that the neck appendages are adsorption organelles in Bacillus subtilis bacteriophage phi29. J Virol. 1978 Sep;27(3):831–834. doi: 10.1128/jvi.27.3.831-834.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno F. Suppressor-sensitive mutants and genetic map of Bacillus subtilis bacteriophage phi 29. Virology. 1974 Nov;62(1):1–16. doi: 10.1016/0042-6822(74)90298-0. [DOI] [PubMed] [Google Scholar]
- Méndez E., Ramírez G., Salas M., Viñuela E. Structural proteins of bacteriophage phi 29. Virology. 1971 Sep;45(3):567–576. doi: 10.1016/0042-6822(71)90172-3. [DOI] [PubMed] [Google Scholar]
- Ramírez G., Méndez E., Salas M., Viñuela E. Head-neck connecting protein in phage phi29. Virology. 1972 Apr;48(1):263–265. doi: 10.1016/0042-6822(72)90134-1. [DOI] [PubMed] [Google Scholar]
- Salas M., Vásquez C., Méndez E., Viñuela E. Head fibers of bacteriophage phi 29. Virology. 1972 Oct;50(1):180–188. doi: 10.1016/0042-6822(72)90358-3. [DOI] [PubMed] [Google Scholar]
- Susskind M. M., Botstein D. Molecular genetics of bacteriophage P22. Microbiol Rev. 1978 Jun;42(2):385–413. doi: 10.1128/mr.42.2.385-413.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurow H., Niemann H., Rudolph C., Stirm S. Host capsule depolymerase activity of bacteriophage particles active on Klebsiella K20 and K24 strains. Virology. 1974 Mar;58(1):306–309. doi: 10.1016/0042-6822(74)90166-4. [DOI] [PubMed] [Google Scholar]
- Tosi M. E., Reilly B. E., Anderson D. L. Morphogenesis of bacteriophage phi29 of Bacillus subtilis: cleavage and assembly of the neck appendage protein. J Virol. 1975 Nov;16(5):1282–1295. doi: 10.1128/jvi.16.5.1282-1295.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi M., Anderson D. L. Antigenic properties of bacteriophage phi 29 structural proteins. J Virol. 1973 Dec;12(6):1548–1559. doi: 10.1128/jvi.12.6.1548-1559.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]


