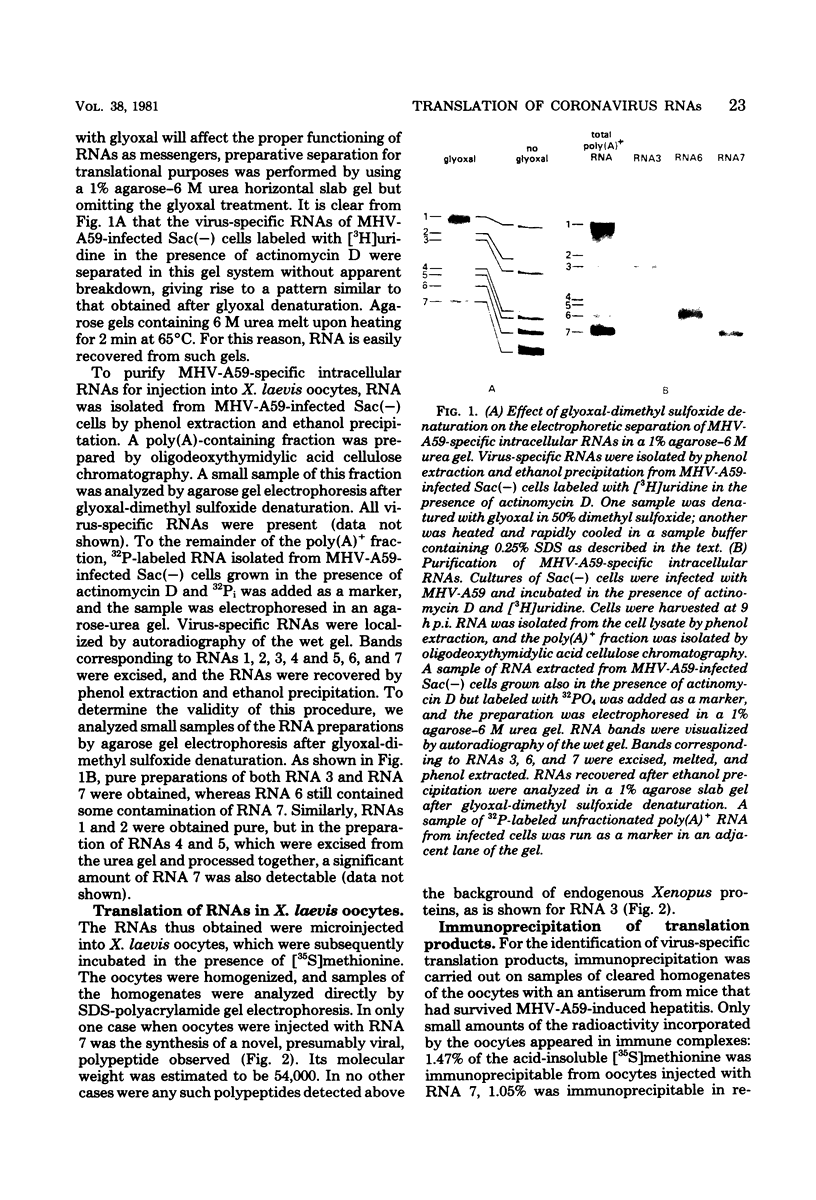
Abstract
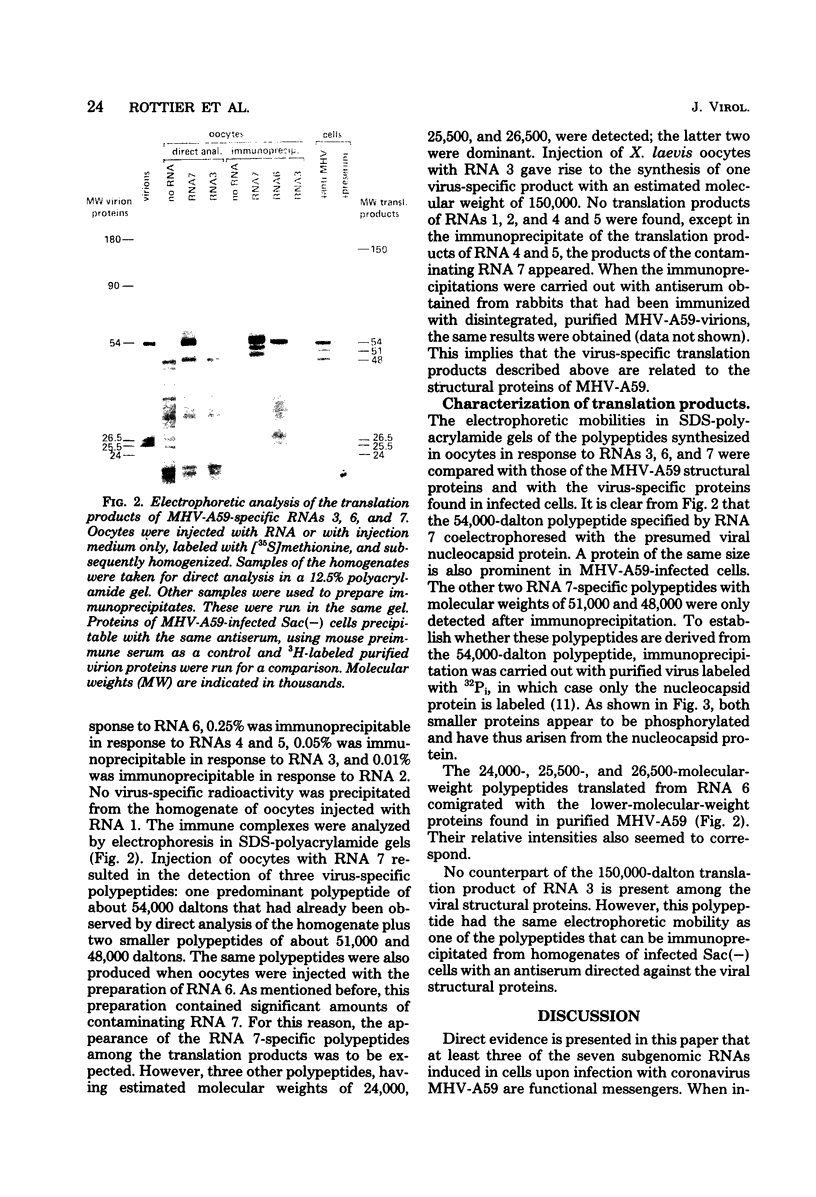
We have purified the seven virus-specific RNAs which were previously shown to be induced in Sac(-) cells upon infection with mouse hepatitis virus strain A59 (W. J. M. Spaan, P. J. M. Rottier, M. C. Horzinek, and B. A. M. van der Zeijst, Virology 108:424-434, 1981). The individual RNAs, prepared by agarose gel electrophoresis of the polyadenylated RNA fraction from infected cells, were obtained pure, except for the preparations of RNAs 4, 5, and 6, which contained some contamination of RNA 7. The RNAs were microinjected into Xenopus laevis oocytes, and after incubation of these cells in the presence of [35S]methionine, the proteins synthesized were analyzed by polyacrylamide gel electrophoresis. Whereas no translation products of RNAs 1, 2, 4, and 5 were detected, the synthesis of virus-specific polypeptides coded by RNAs 3, 6, and 7 was observed. RNA 7 (0.6 X 10(6) daltons) directed the synthesis of a 54,000-molecular-weight polypeptide which comigrated with viral nucleocapsid protein and which was immunoprecipitated by antiserum from mice that had been infected with the virus. RNA 6 (0.9 X 10(6) daltons) directed the synthesis of three polypeptides with molecular weights of 24,000, 25,500, and 26,500, which migrated with the same electrophoretic mobilities as three low-molecular-weight virion polypeptides. After injection of RNA 3 (3.0 X 10(6) daltons), a polypeptide with a molecular weight of about 150,000 was immunoprecipitated. This polypeptide had no counterpart in the virion, but comigrated with a virus-specific glycoprotein present in infected cells which is immunoprecipitated by a rabbit antiserum against the mouse hepatitis virus strain A59 structural proteins. This antiserum could also immunoprecipitate the translation products of RNAs 3, 6, and 7. These results indicate that RNAs 3, 6, and 7 encode viral structural proteins. The significance of the data with respect to the strategy of coronavirus replication is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Cheley S., Haworth-Hatherell E. Comparison of polypeptides of two strains of murine hepatitis virus. Virology. 1979 Sep;97(2):492–494. doi: 10.1016/0042-6822(79)90363-5. [DOI] [PubMed] [Google Scholar]
- Asselbergs F. A., Van Venrooij W. J., Bloemendal H. Synthesis of lens crystallins in Xenopus oocytes as determined by quantitative immunoprecipitation. Eur J Biochem. 1978 Jul 3;87(3):517–524. doi: 10.1111/j.1432-1033.1978.tb12402.x. [DOI] [PubMed] [Google Scholar]
- Bond C. W., Leibowitz J. L., Robb J. A. Pathogenic murine coronaviruses. II. Characterization of virus-specific proteins of murine coronaviruses JHMV and A59V. Virology. 1979 Apr 30;94(2):371–384. doi: 10.1016/0042-6822(79)90468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B. Changes in somatic cell nuclei inserted into growing and maturing amphibian oocytes. J Embryol Exp Morphol. 1968 Nov;20(3):401–414. [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Siddell S. G., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: cell-free synthesis of structural protein p60. J Virol. 1980 Jan;33(1):10–17. doi: 10.1128/jvi.33.1.10-17.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan W. J., Rottier P. J., Horzinek M. C., van der Zeijst B. A. Isolation and identification of virus-specific mRNAs in cells infected with mouse hepatitis virus (MHV-A59). Virology. 1981 Jan 30;108(2):424–434. doi: 10.1016/0042-6822(81)90449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kennedy S. I. Coronavirus multiplication strategy. I. Identification and characterization of virus-specified RNA. J Virol. 1980 Jun;34(3):665–674. doi: 10.1128/jvi.34.3.665-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S. A., Lai M. M. Phosphoproteins of murine hepatitis viruses. J Virol. 1979 Nov;32(2):672–675. doi: 10.1128/jvi.32.2.672-675.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. Characterization of coronavirus II. Glycoproteins of the viral envelope: tryptic peptide analysis. Virology. 1977 Apr;77(2):650–660. doi: 10.1016/0042-6822(77)90489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S. I. Structural proteins: effects of preparative conditions on the migration of protein in polyacrylamide gels. Virology. 1977 Apr;77(2):637–649. doi: 10.1016/0042-6822(77)90488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D. A., Alexander D. J., Almeida J. D., Cunningham C. H., Easterday B. C., Garwes D. J., Hierholzer J. C., Kapikian A., Macnaughton M. R., McIntosh K. Coronaviridae: second report. Intervirology. 1978;10(6):321–328. doi: 10.1159/000148996. [DOI] [PMC free article] [PubMed] [Google Scholar]