Abstract
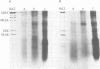
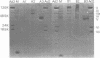
Plasma membranes from HeLa cells were isolated in a two-phase polymer system. To compare the efficiency of attachment protein extraction, a normalized assay for the assessment of adenovirus type 2 (Ad2) receptor-active components interfering with the attachment of Ad2 to HeLa cells was developed. An optimized detergent extraction procedure, 0.5% Triton X-100, was used, and solubilized membrane proteins were radioisotope labeled in vitro. Proteins with affinity for Ad2 virions were quantified and identified in a sucrose gradient sedimentation assay and by affinity chromatography with cross-linked Ad2 virions immobilized to AH-Sepharose 4B. From virions recovered in the sucrose gradient system, one major membrane component of high affinity was identified with a polypeptide molecular weight of around 40,000. Glycosylated proteins isolated by wheat germ lectin chromatography with high affinity for immobilized virus particles were isolated, and two major components with apparent molecular weights of 40,000 and 42,000 were identified. We suggest that a glycosylated protein with high affinity for Ad2 virions and a polypeptide molecular weight of 40,000 to 42,000 is one component of the Ad2 attachment site on HeLa cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Blumenfeld O. O. The proteins of the erythrocyte membrane obtained by solubilization with aqueous pyridine solution. Biochem Biophys Res Commun. 1968 Jan 25;30(2):200–205. doi: 10.1016/0006-291x(68)90471-3. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B., Hagopian A., Eylar E. H. Cellular membranes: the isolation and characterization of the plasma and smooth membranes of HeLa cells. Arch Biochem Biophys. 1968 Oct;128(1):51–69. doi: 10.1016/0003-9861(68)90008-8. [DOI] [PubMed] [Google Scholar]
- Brake E. T., Will P. C., Cook J. S. Characterization of HeLa 5'-nucleotidase: a stable plasma membrane marker. Membr Biochem. 1978;2(1):17–46. doi: 10.3109/09687687809063856. [DOI] [PubMed] [Google Scholar]
- Chen K. Y., Kramer R. H., Canellakis E. S. Isolation and characterization of surface glycoproteins from L-1210, P-388 and HeLa cells. Biochim Biophys Acta. 1978 Feb 2;507(1):107–118. doi: 10.1016/0005-2736(78)90378-4. [DOI] [PubMed] [Google Scholar]
- Dorsett P. H., G8nsberg H. S. Characterization of type 5 adenovirus fiber protein. J Virol. 1975 Jan;15(1):208–216. doi: 10.1128/jvi.15.1.208-216.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dottavio-Martin D., Ravel J. M. Radiolabeling of proteins by reductive alkylation with [14C]formaldehyde and sodium cyanoborohydride. Anal Biochem. 1978 Jul 1;87(2):562–565. doi: 10.1016/0003-2697(78)90706-6. [DOI] [PubMed] [Google Scholar]
- Everitt E., Meador S. A., Levine A. S. Synthesis and processing of the precursor to the major core protein of adenovirus type 2. J Virol. 1977 Jan;21(1):199–214. doi: 10.1128/jvi.21.1.199-214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Philipson L. Mechanism of the arginine requirement for adenovirus synthesis. I. Synthesis of structural proteins. J Virol. 1971 Nov;8(5):742–753. doi: 10.1128/jvi.8.5.742-753.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefrath S. P., Reynolds J. A. The molecular weight of the major glycoprotein from the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3913–3916. doi: 10.1073/pnas.71.10.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon G., Roy C., Jard S. A systematic study of effects of non-ionic detergens on solubilization and activity of pig kidney adenylate cyclase. Eur J Biochem. 1978 Dec;92(2):341–348. doi: 10.1111/j.1432-1033.1978.tb12753.x. [DOI] [PubMed] [Google Scholar]
- HEPPEL L. A., HILMORE R. J. Purification and properties of 5-nucleotidase. J Biol Chem. 1951 Feb;188(2):665–676. [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hennache B., Boulanger P. Biochemical study of KB-cell receptor for adenovirus. Biochem J. 1977 Aug 15;166(2):237–247. doi: 10.1042/bj1660237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrycay E. G., O'Brien P. J. Microsomal electron transport. II. Reduced nicotinamide adenine dinucleotide--cytochrome b5 reductase and cytochrome P-450 as electron carriers in microsomal NADH-peroxidase activity. Arch Biochem Biophys. 1974 Jan;160(1):230–245. doi: 10.1016/s0003-9861(74)80030-5. [DOI] [PubMed] [Google Scholar]
- Jay G., Jay F. T., Friedman R. M., Levine A. S. Simian virus 40-specific ribosome-binding proteins induced by a nondefective adenovirus 2-simian virus 40 hybrid. J Virol. 1977 Sep;23(3):692–699. doi: 10.1128/jvi.23.3.692-699.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen S., Stokke T., Prydz H. HeLa cell plasma membranes. I. 5'-Nucleotidase and ouabain-sensitive ATPase as markers for plasma membranes. J Cell Biol. 1974 Nov;63(2 Pt 1):357–363. doi: 10.1083/jcb.63.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A. Early interactions of viruses with cellular membranes. Adv Virus Res. 1979;24:223–276. doi: 10.1016/s0065-3527(08)60395-4. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Wrigley N. G., Pereira H. G. Removal of pentons from particles of adenovirus type 2. Virology. 1969 Nov;39(3):599–604. doi: 10.1016/0042-6822(69)90111-1. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early interaction between animal viruses and cells. Monogr Virol. 1974;9:1–148. [PubMed] [Google Scholar]
- Lotan R., Nicolson G. L. Purification of cell membrane glycoproteins by lectin affinity chromatography. Biochim Biophys Acta. 1979 Dec 20;559(4):329–376. doi: 10.1016/0304-4157(79)90010-8. [DOI] [PubMed] [Google Scholar]
- Maddy A. H. The properties of the protein of the plasma membrane of ox erythrocytes. Biochim Biophys Acta. 1966 Mar 28;117(1):193–200. doi: 10.1016/0304-4165(66)90166-8. [DOI] [PubMed] [Google Scholar]
- Meager A., Butters T. D., Mautner V., Hughes R. C. Interactions of KB-cell glycoproteins with an adenovirus capsid protein. Eur J Biochem. 1976 Jan 15;61(2):345–353. doi: 10.1111/j.1432-1033.1976.tb10028.x. [DOI] [PubMed] [Google Scholar]
- Oh S. K., Pellegrino M. A., Reisfeld R. A. Hypertonic salt extraction of HL-A antigens: assessment of protease activity. Proc Soc Exp Biol Med. 1974 Apr;145(4):1272–1277. doi: 10.3181/00379727-145-37995. [DOI] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prage L., Pettersson U., Höglund S., Lonberg-Holm K., Philipson L. Structural proteins of adenoviruses. IV. Sequential degradation of the adenovirus type 2 virion. Virology. 1970 Oct;42(2):341–358. doi: 10.1016/0042-6822(70)90278-3. [DOI] [PubMed] [Google Scholar]
- Reisfeld R. A., Pellegrino M. A., Kahan B. D. Salt extraction of soluble HL-A antigens. Science. 1971 Jun 11;172(3988):1134–1136. doi: 10.1126/science.172.3988.1134. [DOI] [PubMed] [Google Scholar]
- Sunquist B., Pettersson U., Thelander L., Philipson L. Structural proteins of adenoviruses. IX. Molecular weight and subunit composition of adenovirus type 2 fiber. Virology. 1973 Jan;51(1):252–256. doi: 10.1016/0042-6822(73)90389-9. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Pereira H. G. Antigens and structure of the adenovirus. J Mol Biol. 1965 Aug;13(1):13–20. doi: 10.1016/s0022-2836(65)80076-6. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., van Deenen L. L. The solubilization of human erythrocyte membranes by n-pentanol. Biochim Biophys Acta. 1968 Mar 1;150(2):323–325. doi: 10.1016/0005-2736(68)90179-x. [DOI] [PubMed] [Google Scholar]