Abstract
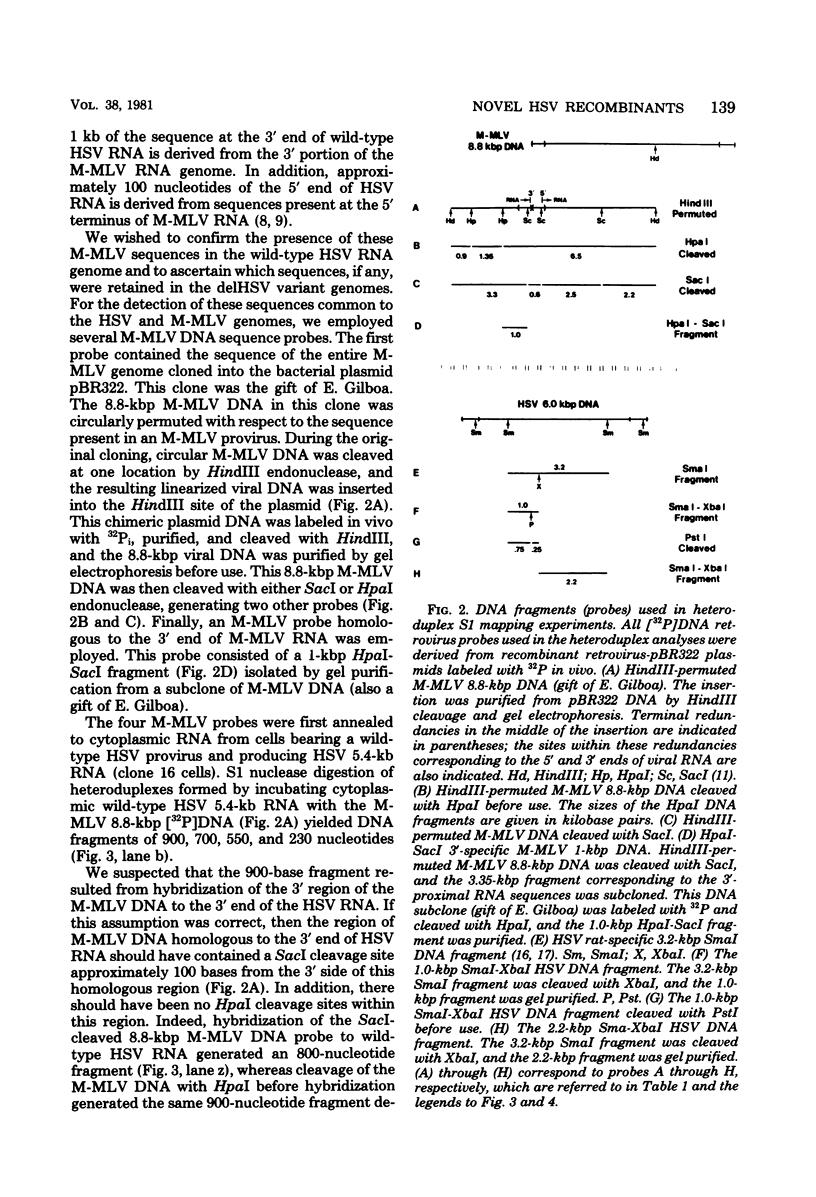
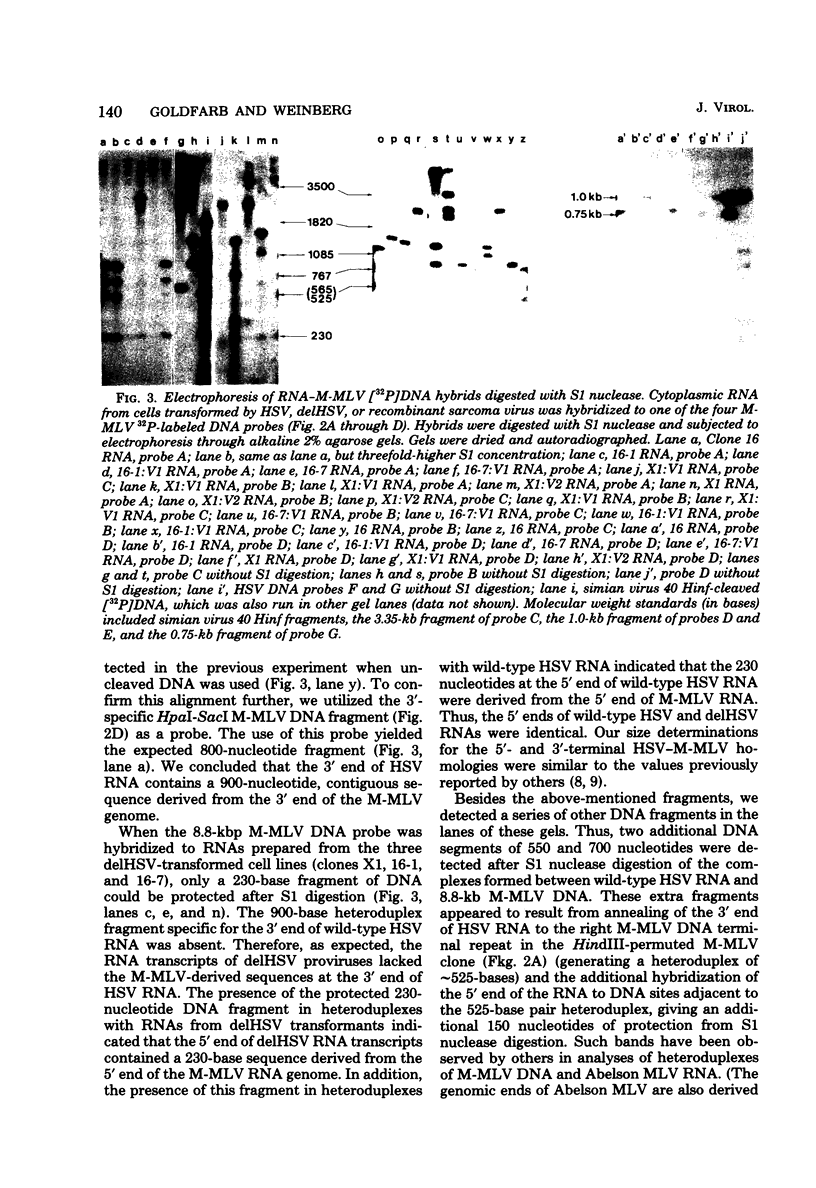
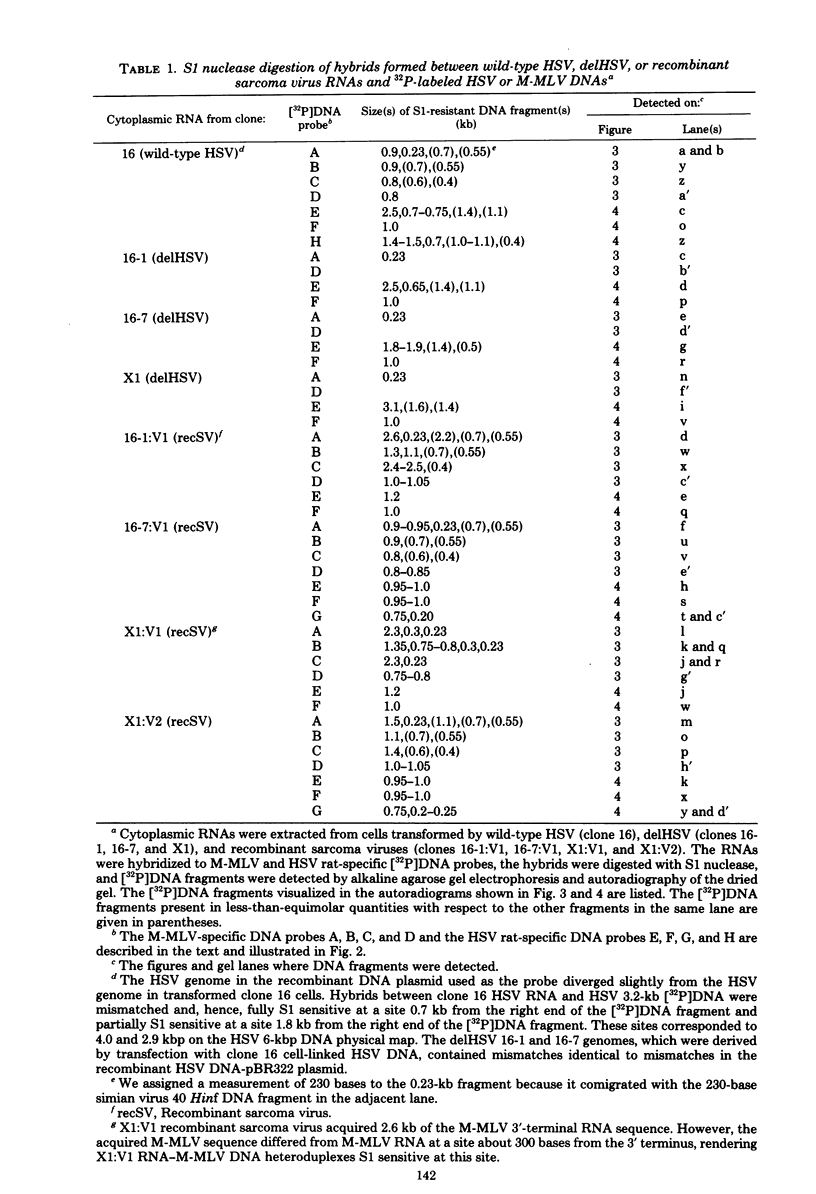
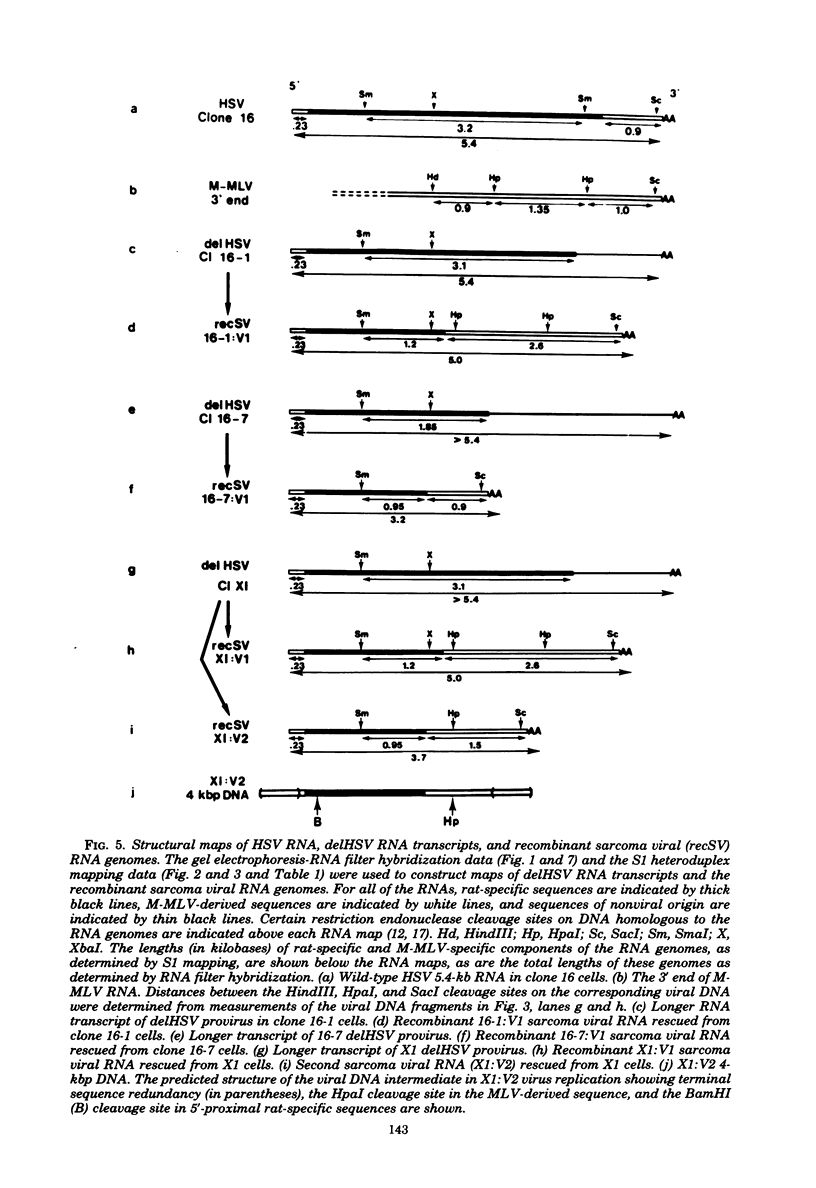
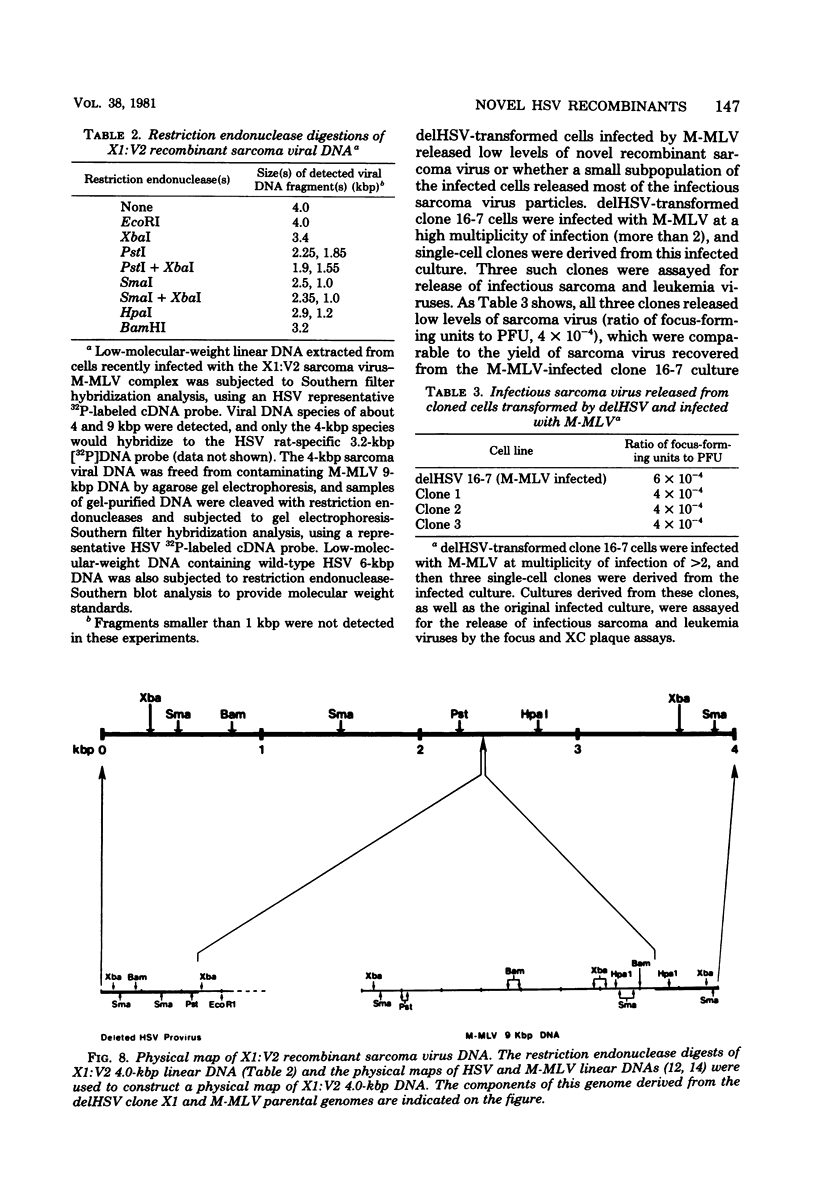
NIH 3T3 cells transfected with Harvey sarcoma virus (HSV) DNA may acquire deleted proviruses (Goldfarb and Weinberg, J. Virol. 38:125-135, 1981). Such proviruses lack the right end of the wild-type HSV DNA genome corresponding to the 3'-proximal portion of the viral RNA. As expected, the RNA transcripts of these deleted HSV (delHSV) proviruses lacked sequences normally found at the 3' end of wild-type HSV RNA. Since frequently these delHSV RNA transcripts were longer than wild-type HSV RNA, we suggest that transcription proceeded through the deleted provirus and continued into flanking nonviral sequences. When delHSV-transformed cells were infected with Moloney murine leukemia virus (M-MLV), delHSV RNA was pseudotyped into new virus particles, demonstrating that the 3'-proximal sequences of wild-type HSV RNA are not essential for virion RNA encapsidation. Cells which carried a delHSV genome and were infected with M-MLV helper released very low titers of highly transmissible sarcoma virus. The inability to rescue high titers of sarcoma virus from these cells reflected the necessary presence of the deleted 3'-terminal sequences for normal efficient transmission of the sarcoma virus genome (Goldfarb and Weinberg, J. Virol. 38:125-135, 1981). The small amount of highly transmissible sarcoma virus rescuable from delHSV-transformed cells originated via genetic recombination between del HSV and the M-MLV helper used for the sarcoma virus rescue. The recombinant sarcoma virus genomes reacquired a competent 3' genomic end from the parental M-MLV genome, which restored efficient transmissibility. The locations of sites for recombination between the delHSV and M-MLV genomes appeared to be nonrandom. These sites were in genomic regions where the parental genomes bore no detectable sequence homology. Structural mapping of these recombinant sarcoma virus genomes indicated that the HSV transformation gene lies within 2.0 kilobases of the RNA 5' end. Based upon our genetic recombination studies, we suggest a model to explain how leukemia viruses can recombine with cellular sequences to generate novel defective viruses.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson P., Goldfarb M. P., Weinberg R. A. A defined subgenomic fragment of in vitro synthesized Moloney sarcoma virus DNA can induce cell transformation upon transfection. Cell. 1979 Jan;16(1):63–75. doi: 10.1016/0092-8674(79)90188-0. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Besmer P., Fan H., Paskind M., Baltimore D. Isolation and characterization of a mouse cell line containing a defective Moloney murine leukemia virus genome. J Virol. 1979 Mar;29(3):1023–1034. doi: 10.1128/jvi.29.3.1023-1034.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Maryak J. M., Wei C. M., Shih T. Y., Shober R., Cheung H. L., Ellis R. W., Hager G. L., Scolnick E. M., Lowy D. R. Functional organization of the Harvey murine sarcoma virus genome. J Virol. 1980 Jul;35(1):76–92. doi: 10.1128/jvi.35.1.76-92.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. W., Garon C. F., Chang E. H., Lowy D. R., Hager G. L., Scolnick E. M., Repaske R., Martin M. A. Molecular cloning of the Harvey sarcoma virus circular DNA intermediates. II. Further structural analyses. J Virol. 1980 Feb;33(2):845–855. doi: 10.1128/jvi.33.2.845-855.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien U. H., Lai M., Shih T. Y., Verma I. M., Scolnick E. M., Roy-Burman P., Davidson N. Heteroduplex analysis of the sequence relationships between the genomes of Kirsten and Harvey sarcoma viruses, their respective parental murine leukemia viruses, and the rat endogenous 30S RNA. J Virol. 1979 Sep;31(3):752–760. doi: 10.1128/jvi.31.3.752-760.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Hageman T. C., Maxam A. M., Haseltine W. A. Structure of the genome of Moloney murine leukemia virus: a terminally redundant sequence. Cell. 1978 Apr;13(4):761–773. doi: 10.1016/0092-8674(78)90226-x. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Goff S., Shields A., Yoshimura F., Mitra S., Baltimore D. In vitro synthesis of a 9 kbp terminally redundant DNA carrying the infectivity of Moloney murine leukemia virus. Cell. 1979 Apr;16(4):863–874. doi: 10.1016/0092-8674(79)90101-6. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Mitra S. W., Goff S., Baltimore D. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979 Sep;18(1):93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Goldberg R. J., Levin R., Parks W. P., Scolnick E. M. Quantitative analysis of the rescue of RNA sequences by mammalian type C viruses. J Virol. 1975 Jan;17(1):43–50. doi: 10.1128/jvi.17.1.43-50.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. P., Weinberg R. A. Physical map of biologically active Harvey sarcoma virus unintegrated linear DNA. J Virol. 1979 Oct;32(1):30–39. doi: 10.1128/jvi.32.1.30-39.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. P., Weinberg R. A. Structure of the provirus within NIH 3T3 cells transfected with Harvey sarcoma virus DNA. J Virol. 1981 Apr;38(1):125–135. doi: 10.1128/jvi.38.1.125-135.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY J. J. AN UNIDENTIFIED VIRUS WHICH CAUSES THE RAPID PRODUCTION OF TUMOURS IN MICE. Nature. 1964 Dec 12;204:1104–1105. doi: 10.1038/2041104b0. [DOI] [PubMed] [Google Scholar]
- Hager G. L., Chang E. H., Chan H. W., Garon C. F., Israel M. A., Martin M. A., Scolnick E. M., Lowy D. R. Molecular cloning of the Harvey sarcoma virus closed circular DNA intermediates: initial structural and biological characterization. J Virol. 1979 Sep;31(3):795–809. doi: 10.1128/jvi.31.3.795-809.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry. 1973 Jun 5;12(12):2330–2338. doi: 10.1021/bi00736a023. [DOI] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Medeiros E., Hayward W. S. An avian oncovirus mutant (SE 21Q1b) deficient in genomic RNA: biological and biochemical characterization. Cell. 1978 Dec;15(4):1371–1381. doi: 10.1016/0092-8674(78)90062-4. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P. Harvey sarcoma virus: a second murine type C sarcoma virus with rat genetic information. J Virol. 1974 Jun;13(6):1211–1219. doi: 10.1128/jvi.13.6.1211-1219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Rands E., Williams D., Parks W. P. Studies on the nucleic acid sequences of Kirsten sarcoma virus: a model for formation of a mammalian RNA-containing sarcoma virus. J Virol. 1973 Sep;12(3):458–463. doi: 10.1128/jvi.12.3.458-463.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Gallimore P. H., Flint S. J. Mapping of adenovirus 2 RNA sequences in lytically infected cells and transformed cell lines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):457–474. doi: 10.1101/sqb.1974.039.01.058. [DOI] [PubMed] [Google Scholar]
- Shields A., Goff S., Paskind M., Otto G., Baltimore D. Structure of the Abelson murine leukemia virus genome. Cell. 1979 Dec;18(4):955–962. doi: 10.1016/0092-8674(79)90208-3. [DOI] [PubMed] [Google Scholar]
- Shields A., Witte W. N., Rothenberg E., Baltimore D. High frequency of aberrant expression of Moloney murine leukemia virus in clonal infections. Cell. 1978 Jul;14(3):601–609. doi: 10.1016/0092-8674(78)90245-3. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scholnick E. M. Identification of a sarcoma virus-coded phosphoprotein in nonproducer cells transformed by Kirsten or Harvey murine sarcoma virus. Virology. 1979 Jul 15;96(1):64–79. doi: 10.1016/0042-6822(79)90173-9. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scolnick E. M. p21 of Kirsten murine sarcoma virus is thermolabile in a viral mutant temperature sensitive for the maintenance of transformation. J Virol. 1979 Aug;31(2):546–546. doi: 10.1128/jvi.31.2.546-546.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]