Abstract
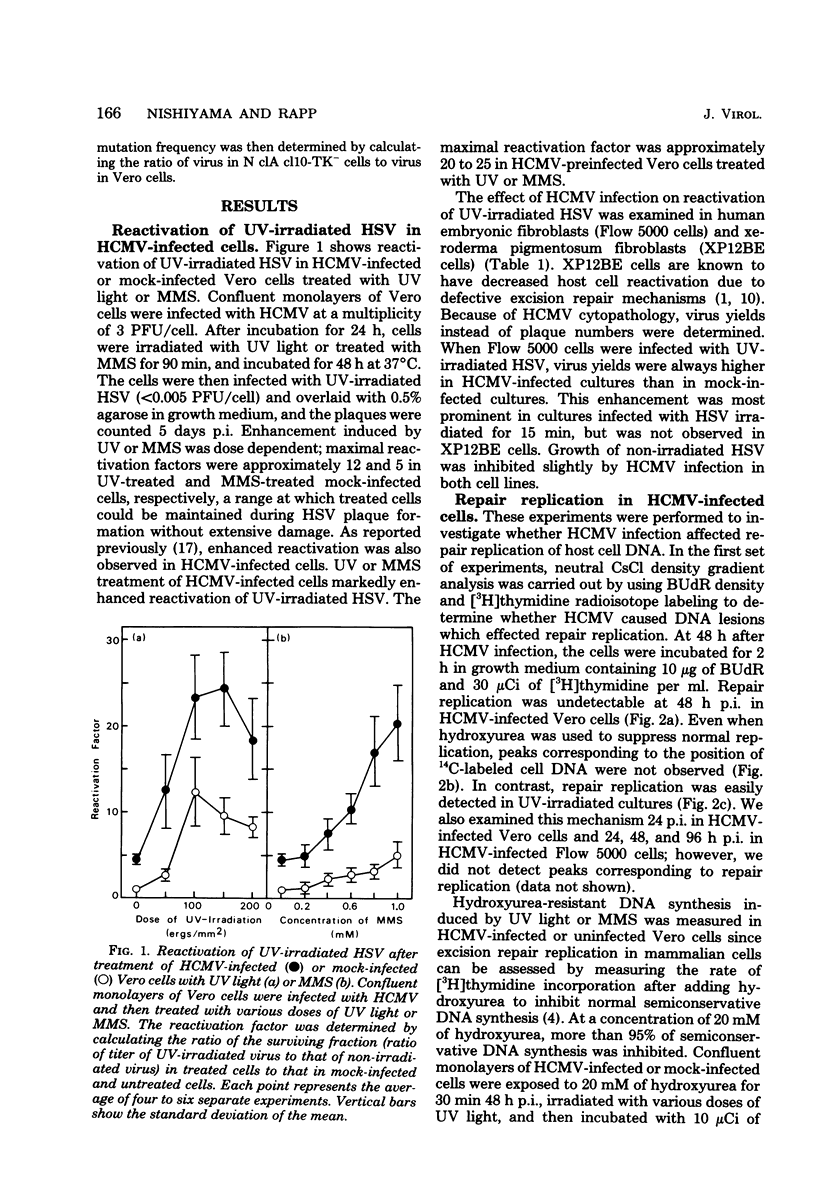
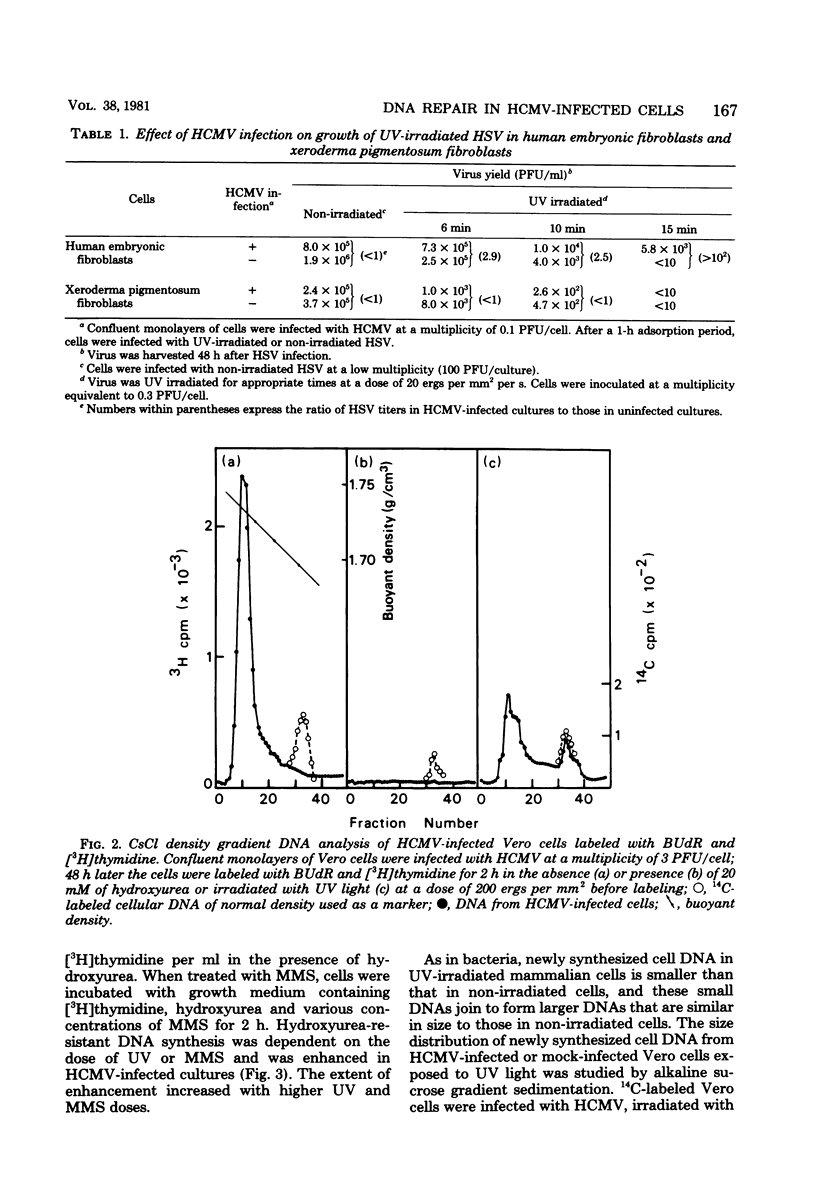
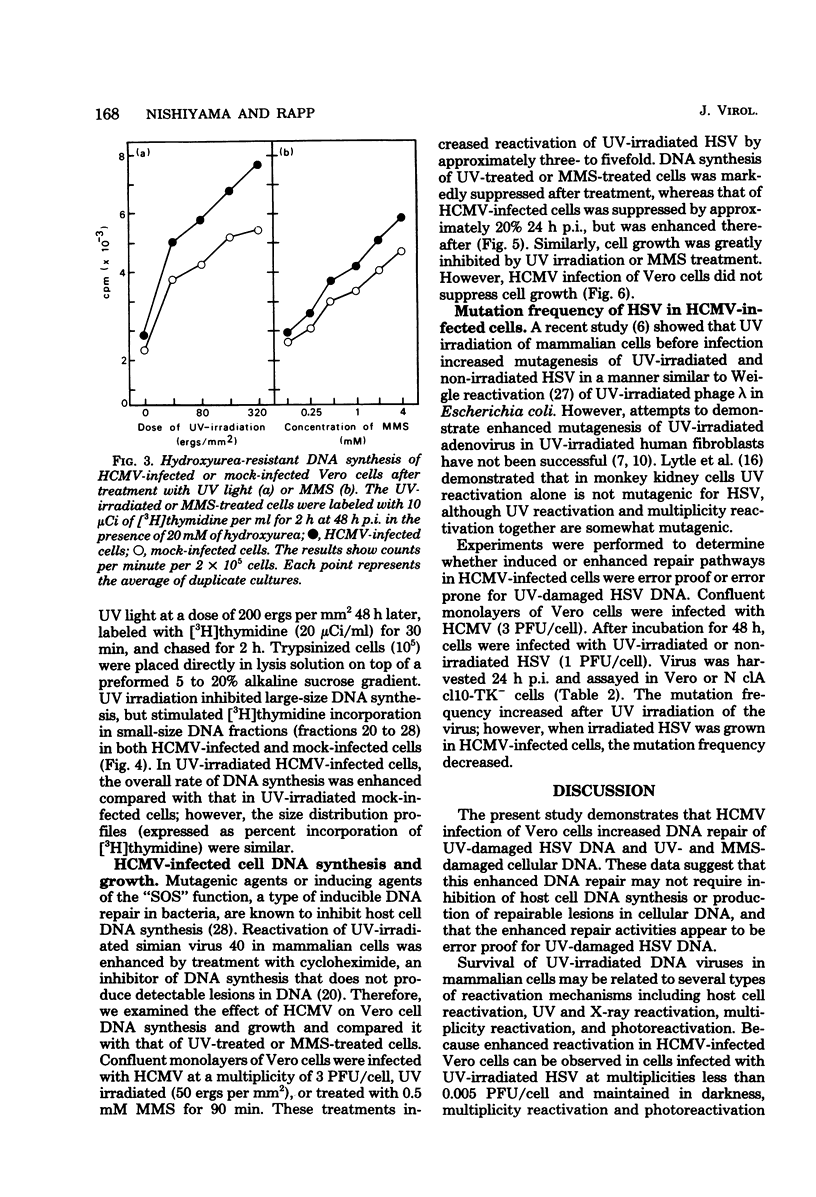
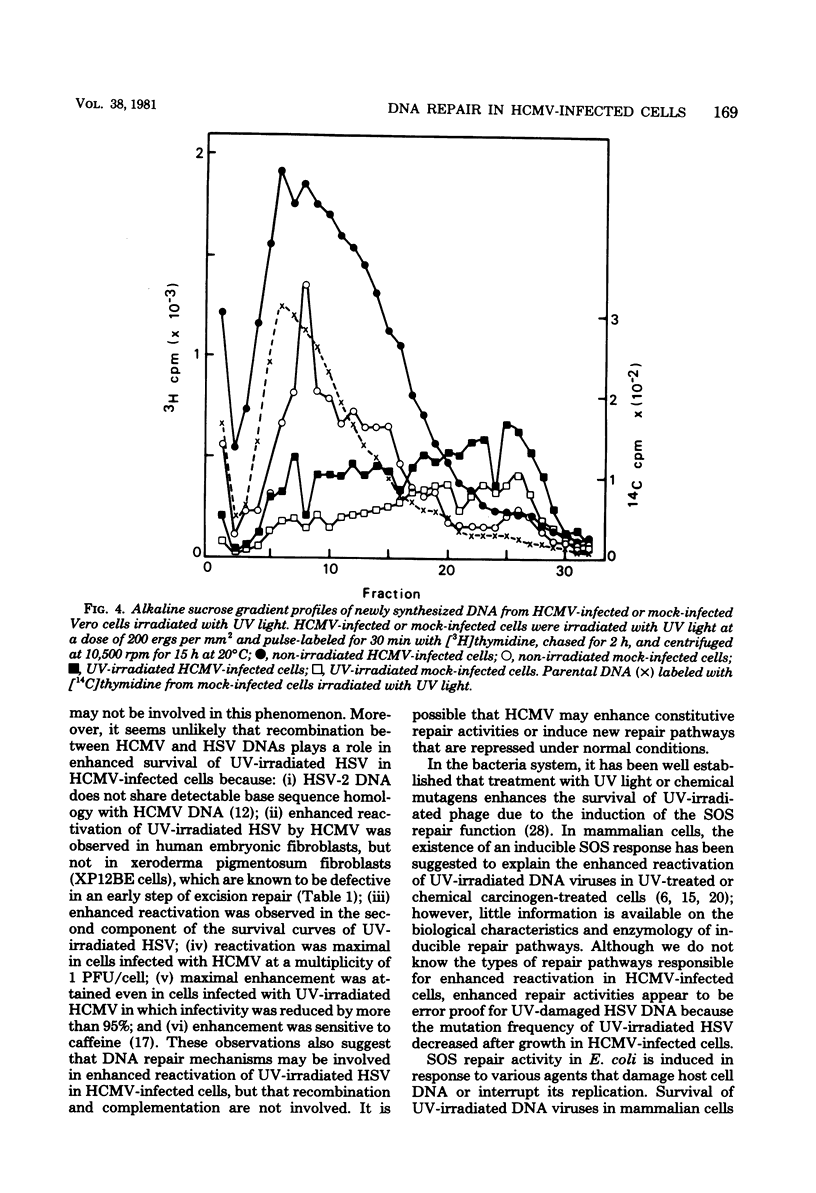
Plaque formation in Vero cells by UV-irradiated herpes simplex virus was enhanced by infection with human cytomegalovirus (HCMV), UV irradiation, or treatment with methylmethanesulfonate. Preinfection of Vero cells with HCMV enhanced reactivation of UV-irradiated herpes simplex virus more significantly than did treatment with UV or methylmethanesulfonate alone. A similar enhancement by HCMV was observed in human embryonic fibroblasts, but not in xeroderma pigmentosum (XP12BE) cells. It was also found that HCMV infection enhanced hydroxyurea-resistant DNA synthesis induced by UV light or methylmethanesulfonate. Alkaline sucrose gradient sedimentation analysis revealed an enhanced rate of synthesis of all size classes of DNA in UV-irradiated HCMV-infected Vero cells. However, HCMV infection did not induce repairable lesions in cellular DNA and did not significantly inhibit host cell DNA synthesis, unlike UV or methylmethanesulfonate. These results indicate that HCMV enhanced DNA repair capacity in the host cells without producing detectable lesions in cellular DNA and without inhibiting DNA synthesis. This repair appeared to be error proof for UV-damaged herpes simplex virus DNA when tested with herpes simplex virus thymidine kinase-negative mutants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Lytle C. D. Decreased host cell reactivation of irradiated SV40 virus in xeroderma pigmentosum. Nature. 1970 Oct 24;228(5269):359–361. doi: 10.1038/228359a0. [DOI] [PubMed] [Google Scholar]
- Albrecht T., Rapp F. Malignant transformation of hamster embryo fibroblasts following exposure to ultraviolet-irradiated human cytomegalovirus. Virology. 1973 Sep;55(1):53–61. doi: 10.1016/s0042-6822(73)81007-4. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertazzoni U., Stefanini M., Noy G. P., Giulotto E., Nuzzo F., Falaschi A., Spadari S. Variations of DNA polymerase-alpha and -beta during prolonged stimulation of human lymphocytes. Proc Natl Acad Sci U S A. 1976 Mar;73(3):785–789. doi: 10.1073/pnas.73.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt W. N., Flamm W. G., Bernheim N. J. The value of hydroxyurea in assessing repair synthesis of DNA in HeLa cells. Chem Biol Interact. 1972 Oct;5(5):327–339. doi: 10.1016/0009-2797(72)90072-5. [DOI] [PubMed] [Google Scholar]
- DasGupta U. B., Summers W. C. Ultraviolet reactivation of herpes simplex virus is mutagenic and inducible in mammlian cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2378–2381. doi: 10.1073/pnas.75.5.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Sakuma S., Plotkin S. A. Human cytomegalovirus infection of WI-38 cells stimulates mitochondrial DNA synthesis. Nature. 1976 Jul 29;262(5567):414–416. doi: 10.1038/262414a0. [DOI] [PubMed] [Google Scholar]
- Geder K. M., Lausch R., O'Neill F., Rapp F. Oncogenic transformation of human embryo lung cells by human cytomegalovirus. Science. 1976 Jun 11;192(4244):1134–1137. doi: 10.1126/science.179143. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C., Cooper P. K., Ganesan A. K., Smith C. A. DNA repair in bacteria and mammalian cells. Annu Rev Biochem. 1979;48:783–836. doi: 10.1146/annurev.bi.48.070179.004031. [DOI] [PubMed] [Google Scholar]
- Hirai K., Watanabe Y. Induction of alpha type DNA polymerases in human cytomegalovirus-infected WI-38 cells. Biochim Biophys Acta. 1976 Oct 18;447(3):328–339. doi: 10.1016/0005-2787(76)90056-3. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Pagano J. S. Human cytomegalovirus. II. Lack of relatedness to DNA of herpes simples I and II, Epstein-Barr virus, and nonhuman strains of cytomegalovirus. J Virol. 1974 Mar;13(3):642–645. doi: 10.1128/jvi.13.3.642-645.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom H. C. Stimulation of ornithine decarboxylase by human cytomegalovirus. J Gen Virol. 1979 Feb;42(2):265–278. doi: 10.1099/0022-1317-42-2-265. [DOI] [PubMed] [Google Scholar]
- Jeor S. C., Albrecht T. B., Funk F. D., Rapp F. Stimulation of cellular DNA synthesis by human cytomegalovirus. J Virol. 1974 Feb;13(2):353–362. doi: 10.1128/jvi.13.2.353-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle C. D., Coppey J., Taylor W. D. Enhanced survival of ultraviolet-irradiated herpes simplex virus in carcinogen-pretreated cells. Nature. 1978 Mar 2;272(5648):60–62. doi: 10.1038/272060a0. [DOI] [PubMed] [Google Scholar]
- Lytle C. D., Goddard J. G., Lin C. H. Repair and mutagenesis of herpes simplex virus in UV-irradiated monkey cells. Mutat Res. 1980 Apr;70(2):139–149. doi: 10.1016/0027-5107(80)90153-0. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Rapp F. Enhanced survival of ultraviolet-irradiated herpes simplex virus in human cytomegalovirus-infected cells. Virology. 1980 Jan 15;100(1):189–193. doi: 10.1016/0042-6822(80)90566-8. [DOI] [PubMed] [Google Scholar]
- Rapp F., Geder L., Murasko D., Lausch R., Ladda R., Huang E. S., Webber M. M. Long-term persistence of cytomegalovirus genome in cultured human cells of prostatic origin. J Virol. 1975 Oct;16(4):982–990. doi: 10.1128/jvi.16.4.982-990.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin A. R., Hanawalt P. C. Carcinogens enhance survival of UV-irradiated simian virus 40 in treated monkey kidney cells: induction of a recovery pathway? Proc Natl Acad Sci U S A. 1978 Jan;75(1):346–350. doi: 10.1073/pnas.75.1.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin A. R., Smith C. A., Hanawalt P. C. Repair of DNA in human cells after treatment with activated aflatoxin B1. Cancer Res. 1977 Jun;37(6):1786–1793. [PubMed] [Google Scholar]
- Scudiero D., Norin A., Karran P., Strauss B. DNA excision-repair deficiency of human peripheral blood lymphocytes treated with chemical carcinogens. Cancer Res. 1976 Apr;36(4):1397–1403. [PubMed] [Google Scholar]
- Sirover M. A. Induction of the DNA repair enzyme uracil-DNA glycosylase in stimulated human lymphocytes. Cancer Res. 1979 Jun;39(6 Pt 1):2090–2095. [PubMed] [Google Scholar]
- Stinski M. F. Synthesis of proteins and glycoproteins in cells infected with human cytomegalovirus. J Virol. 1977 Sep;23(3):751–767. doi: 10.1128/jvi.23.3.751-767.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Furukawa T., Plotkin S. A. Human cytomegalovirus stimulates host cell RNA synthesis. J Virol. 1975 Feb;15(2):297–304. doi: 10.1128/jvi.15.2.297-304.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle J. J. Induction of Mutations in a Bacterial Virus. Proc Natl Acad Sci U S A. 1953 Jul;39(7):628–636. doi: 10.1073/pnas.39.7.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi K., Fogel M., Rapp F. Effect of caffeine on the replication of nonirradiated and ultraviolet-irradiated cytomegalovirus. Intervirology. 1978;10(4):241–253. doi: 10.1159/000148987. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Rapp F. Production of plasminogen activator by human and hamster cells infected with human cytomegalovirus. J Virol. 1979 Aug;31(2):415–419. doi: 10.1128/jvi.31.2.415-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Závada V., Erban V., Rezácová D., Vonka V. Thymidine-kinase in cytomegalovirus infected cells. Arch Virol. 1976;52(4):333–339. doi: 10.1007/BF01315622. [DOI] [PubMed] [Google Scholar]


