Abstract
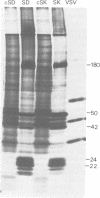
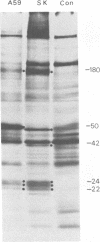
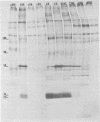
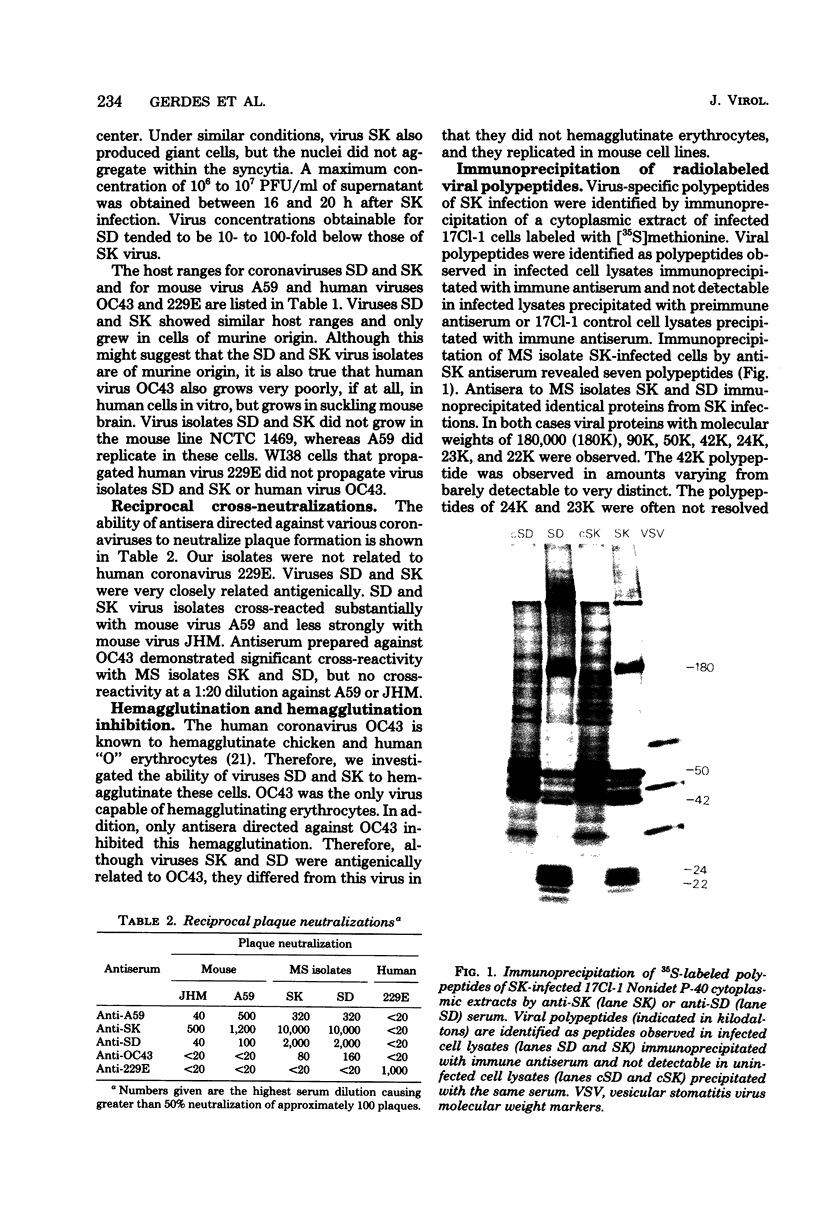
Two coronaviruses (SK and SD), isolated from fresh autopsy brain tissue from two multiple sclerosis patients, were compared with known human and murine coronaviruses. In plaque neutralization assays, antisera prepared against multiple sclerosis isolates SK and SD demonstrated significant cross-reactivity to each other and to murine coronavirus A59, weak cross-reactivity to murine coronavirus JHM, but no cross-reactivity to the human coronavirus 229E. Antiserum to SK or SD failed to inhibit hemagglutination of chicken erythrocytes by the human coronavirus OC43. However, OC43 antiserum neutralized both SD and SK. Specific coronavirus polypeptides were identified and compared by immunoprecipitation and polyacrylamide gel electrophoresis. Infected and mock-infected 17Cl-1 cells were pretreated with actinomycin D and labeled with [35S]methionine. Polypeptides in Nonidet P-40 cytoplasmic extracts were immunoprecipitated with homologous and heterologous antisera. Identical polypeptides were precipitated from A59-, SD-, or SK-infected cell extracts by SD, SK, OC43, or A59 antisera. The polypeptides of human virus 229E were antigenically distinct, with the exception of weak recognition of a polypeptide of 50,000 molecular weight. We conclude that the two multiple sclerosis virus isolates SK and SD are closely related serologically to the murine coronavirus A59 and the human coronavirus OC43.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Cheley S., Haworth-Hatherell E. Comparison of polypeptides of two strains of murine hepatitis virus. Virology. 1979 Sep;97(2):492–494. doi: 10.1016/0042-6822(79)90363-5. [DOI] [PubMed] [Google Scholar]
- Bhatt P. N., Jacoby R. O., Jonas A. M. Respiratory infection in mice with sialodacryoadenitis virus, a coronavirus of rats. Infect Immun. 1977 Dec;18(3):823–827. doi: 10.1128/iai.18.3.823-827.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C. W., Leibowitz J. L., Robb J. A. Pathogenic murine coronaviruses. II. Characterization of virus-specific proteins of murine coronaviruses JHMV and A59V. Virology. 1979 Apr 30;94(2):371–384. doi: 10.1016/0042-6822(79)90468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradburne A. F. Antigenic relationships amongst coronaviruses. Arch Gesamte Virusforsch. 1970;31(3):352–364. doi: 10.1007/BF01253769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks J. S., DeVald B. L., Jankovsky L. D., Gerdes J. C. Two coronaviruses isolated from central nervous system tissue of two multiple sclerosis patients. Science. 1980 Aug 22;209(4459):933–934. doi: 10.1126/science.7403860. [DOI] [PubMed] [Google Scholar]
- Garwes D. J., Pocock D. H. The polypeptide structure of transmissible gastroenteritis virus. J Gen Virol. 1975 Oct;29(1):25–34. doi: 10.1099/0022-1317-29-1-25. [DOI] [PubMed] [Google Scholar]
- HARTLEY J. W., ROWE W. P., BLOOM H. H., TURNER H. C. ANTIBODIES TO MOUSE HEPATITIS VIRUSES IN HUMAN SERA. Proc Soc Exp Biol Med. 1964 Feb;115:414–418. doi: 10.3181/00379727-115-28928. [DOI] [PubMed] [Google Scholar]
- Hajer I., Storz J. Structural polypeptides of the enteropathogenic bovine coronavirus strain LY-138. Arch Virol. 1979;59(1-2):47–57. doi: 10.1007/BF01317894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamre D., Procknow J. J. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966 Jan;121(1):190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon R. M., Griffin D. E., McCormick U., Weiner L. P. Mouse hepatitis virus-induced recurrent demyelination. A preliminary report. Arch Neurol. 1975 Jan;32(1):32–35. doi: 10.1001/archneur.1975.00490430054008. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Broderson J. R., Murphy F. A. New strain of mouse hepatitis virus as the cause of lethal enteritis in infant mice. Infect Immun. 1979 May;24(2):508–522. doi: 10.1128/iai.24.2.508-522.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Palmer E. L., Whitfield S. G., Kaye H. S., Dowdle W. R. Protein composition of coronavirus OC 43. Virology. 1972 May;48(2):516–527. doi: 10.1016/0042-6822(72)90062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C. Purification and biophysical properties of human coronavirus 229E. Virology. 1976 Nov;75(1):155–165. doi: 10.1016/0042-6822(76)90014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Suggs M. T., Hall E. C. Standardized viral hemagglutination and hemagglutination-inhibition tests. II. Description and statistical evaluation. Appl Microbiol. 1969 Nov;18(5):824–833. doi: 10.1128/am.18.5.824-833.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano N., Fujiwara K., Hino S., Matumoto M. Replication and plaque formation of mouse hepatitis virus (MHV-2) in mouse cell line DBT culture. Arch Gesamte Virusforsch. 1974;44(3):298–302. doi: 10.1007/BF01240618. [DOI] [PubMed] [Google Scholar]
- Kaye H. S., Dowdle W. R. Some characteristics of hemagglutination of certain strains of "IBV-like" virus. J Infect Dis. 1969 Nov;120(5):576–581. doi: 10.1093/infdis/120.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Stohlman S. A. RNA of mouse hepatitis virus. J Virol. 1978 May;26(2):236–242. doi: 10.1128/jvi.26.2.236-242.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnaughton M. R. The polypeptides of human and mouse coronaviruses. Brief report. Arch Virol. 1980;63(1):75–80. doi: 10.1007/BF01320763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Becker W. B., Chanock R. M. Growth in suckling-mouse brain of "IBV-like" viruses from patients with upper respiratory tract disease. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2268–2273. doi: 10.1073/pnas.58.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Chao R. K., Krause H. E., Wasil R., Mocega H. E., Mufson M. A. Coronavirus infection in acute lower respiratory tract disease of infants. J Infect Dis. 1974 Nov;130(5):502–507. doi: 10.1093/infdis/130.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Dees J. H., Becker W. B., Kapikian A. Z., Chanock R. M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci U S A. 1967 Apr;57(4):933–940. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Kapikian A. Z., Hardison K. A., Hartley J. W., Chanock R. M. Antigenic relationships among the coronaviruses of man and between human and animal coronaviruses. J Immunol. 1969 May;102(5):1109–1118. [PubMed] [Google Scholar]
- Nagashima K., Wege H., Meyermann R., ter Meulen V. Corona virus induced subacute demyelinating encephalomyelitis in rats: a morphological analysis. Acta Neuropathol. 1978 Oct 13;44(1):63–70. doi: 10.1007/BF00691641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. C., Cross S. S., Rowe W. P. Rat coronavirus (RCV): a prevalent, naturally occurring pneumotropic virus of rats. Arch Gesamte Virusforsch. 1970;31(3):293–302. doi: 10.1007/BF01253764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N. C. Morphologic and physical characteristics of feline infectious peritonitis virus and its growth in autochthonous peritoneal cell cultures. Am J Vet Res. 1976 May;37(5):567–572. [PubMed] [Google Scholar]
- Pedersen N. C., Ward J., Mengeling W. L. Antigenic relationship of the feline infectious peritonitis virus to coronaviruses of other species. Arch Virol. 1978;58(1):45–53. doi: 10.1007/BF01315534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., CAPPS W. I. Mouse hepatitis virus infection as a highly contagious, prevalent, enteric infection of mice. Proc Soc Exp Biol Med. 1963 Jan;112:161–165. doi: 10.3181/00379727-112-27980. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Bond C. W. Pathogenic murine coronaviruses. I. Characterization of biological behavior in vitro and virus-specific intracellular RNA of strongly neurotropic JHMV and weakly neurotropic A59V viruses. Virology. 1979 Apr 30;94(2):352–370. doi: 10.1016/0042-6822(79)90467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt O. W., Cooney M. K., Kenny G. E. Plaque assay and improved yield of human coronaviruses in a human rhabdomyosarcoma cell line. J Clin Microbiol. 1979 Jun;9(6):722–728. doi: 10.1128/jcm.9.6.722-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. G., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: cell-free synthesis of structural protein p60. J Virol. 1980 Jan;33(1):10–17. doi: 10.1128/jvi.33.1.10-17.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V., Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J Virol. 1980 Jan;33(1):449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. Characterization of coronavirus II. Glycoproteins of the viral envelope: tryptic peptide analysis. Virology. 1977 Apr;77(2):650–660. doi: 10.1016/0042-6822(77)90489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S. I. Structural proteins: effects of preparative conditions on the migration of protein in polyacrylamide gels. Virology. 1977 Apr;77(2):637–649. doi: 10.1016/0042-6822(77)90488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Takemoto K. K. Enhanced growth of a murine coronavirus in transformed mouse cells. Infect Immun. 1972 Oct;6(4):501–507. doi: 10.1128/iai.6.4.501-507.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R., Iwasaki Y., Koprowski H. Intracisternal virus-like particles in brain of a multiple sclerosis patient. J Neurol Sci. 1976 May;28(1):121–126. doi: 10.1016/0022-510X(76)90053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Wege H., Nagashima K., ter Meulen V. Structural polypeptides of the murine coronavirus JHM. J Gen Virol. 1979 Jan;42(1):37–47. doi: 10.1099/0022-1317-42-1-37. [DOI] [PubMed] [Google Scholar]