Abstract
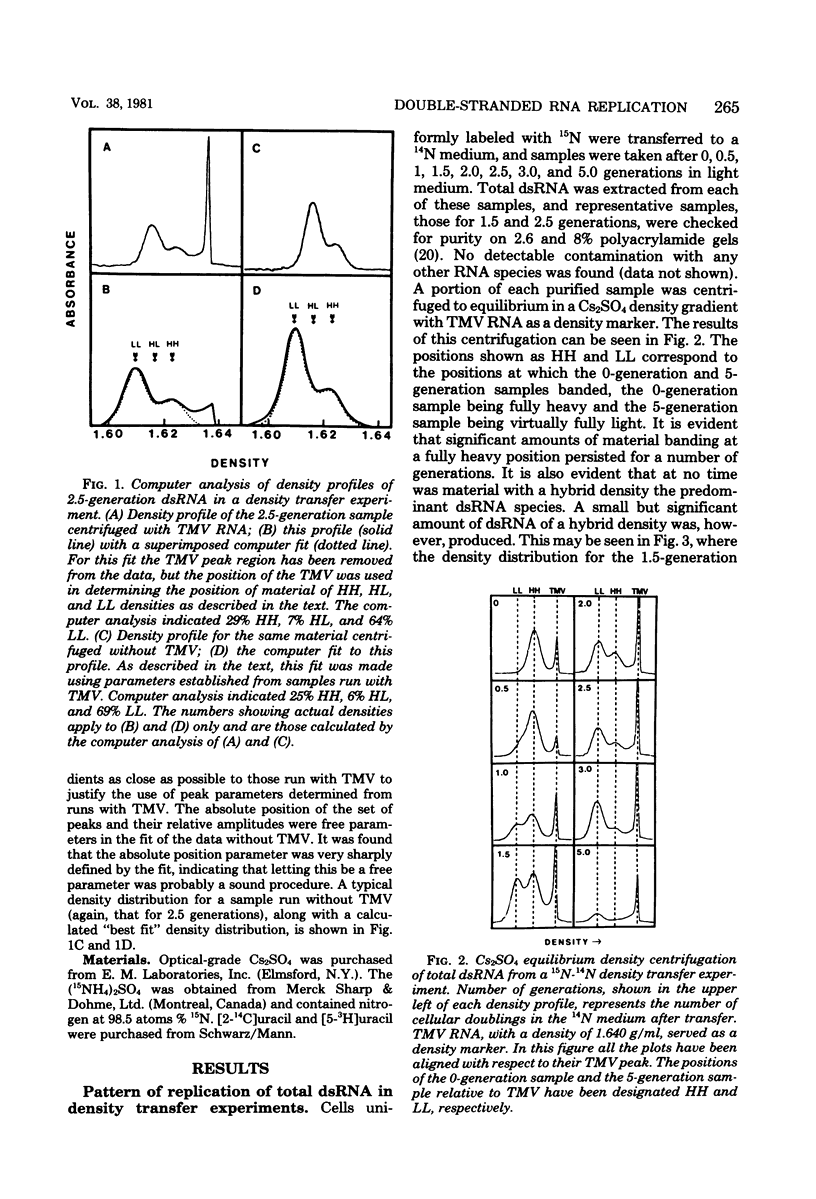
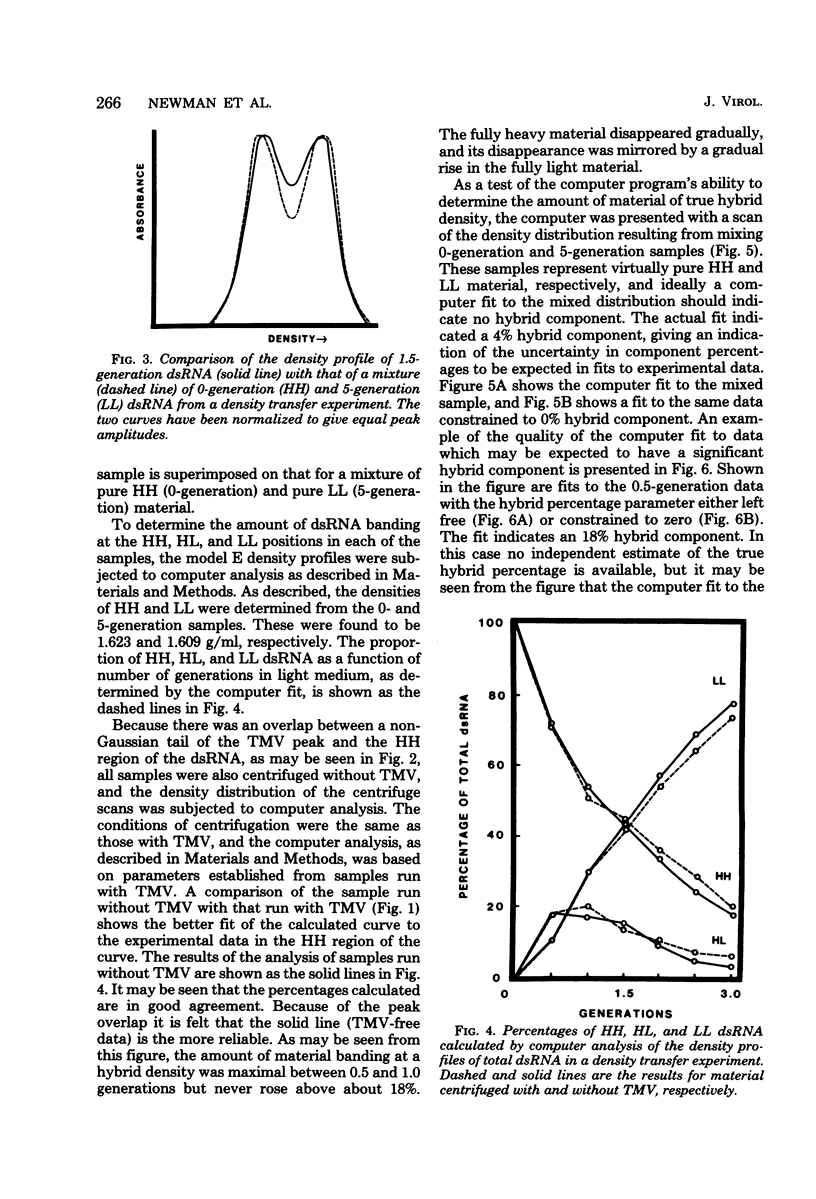
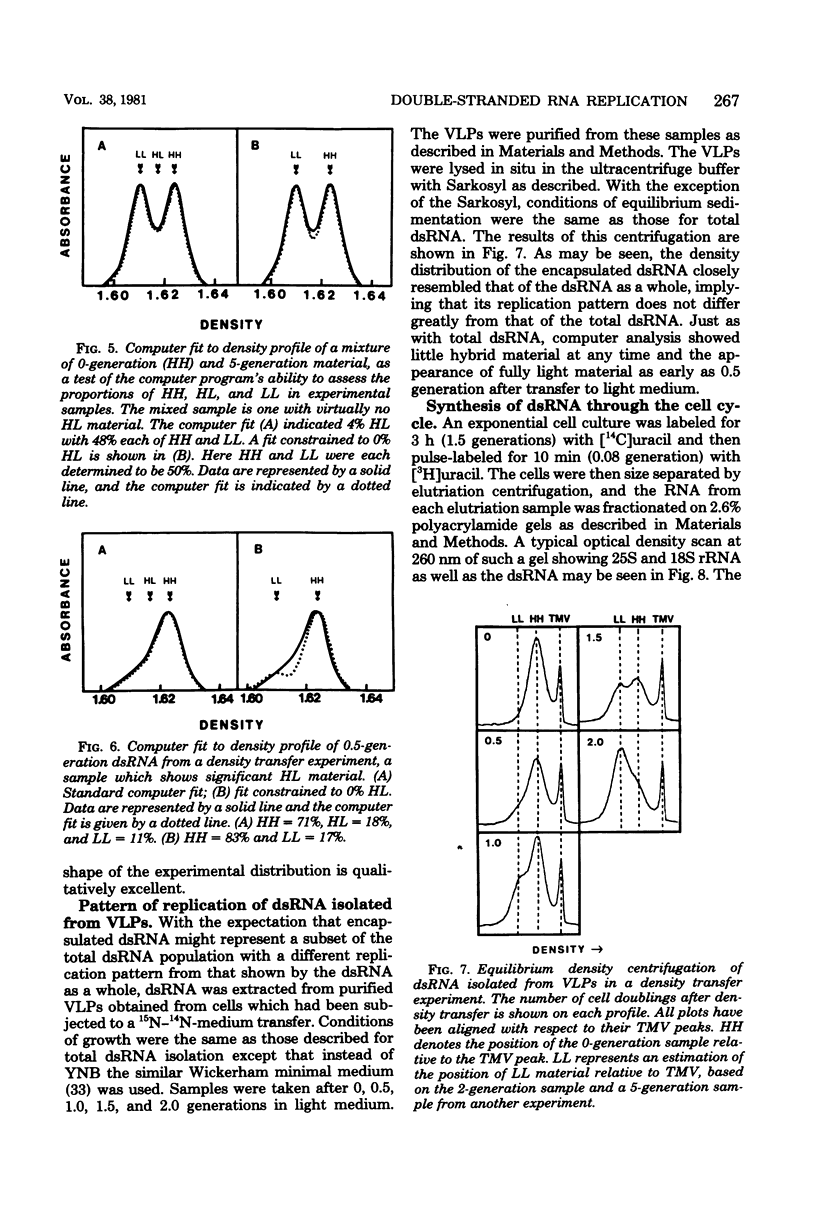
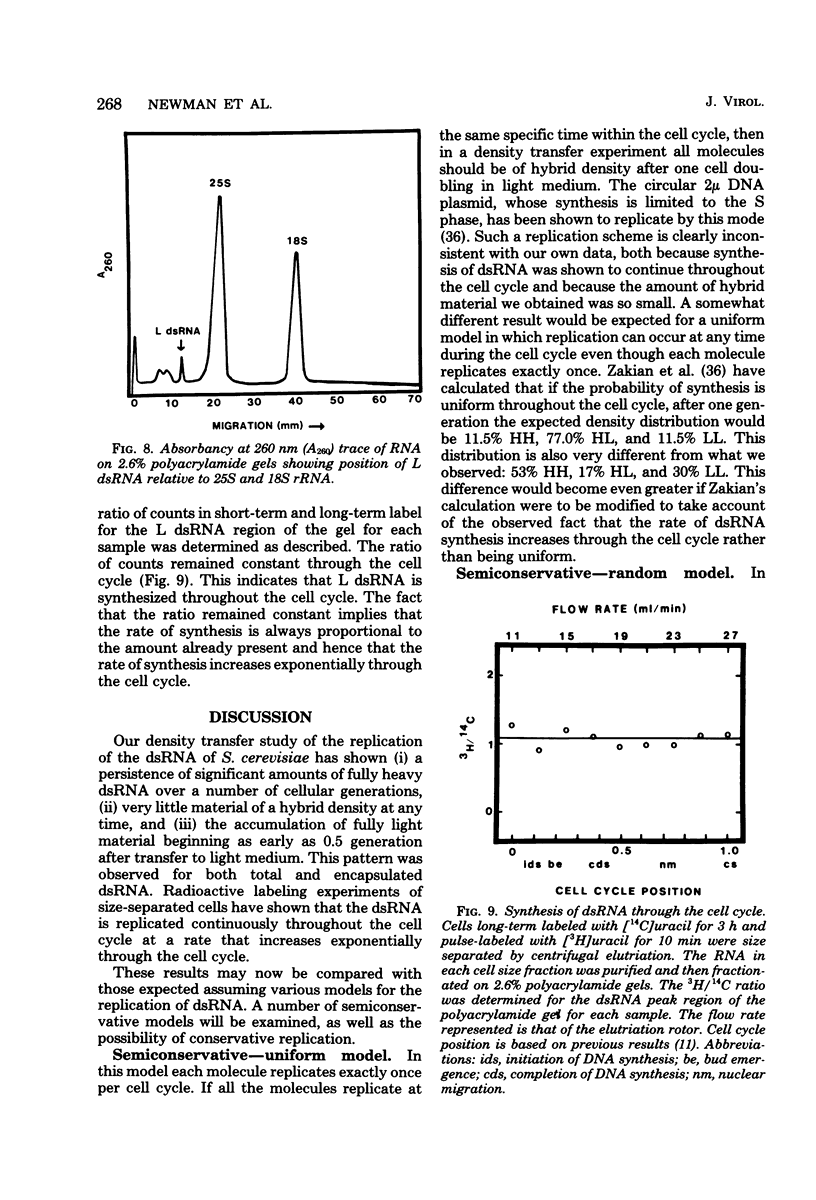
The mode of replication of the L double-stranded RNA (dsRNA) present in virus-like particles in Saccharomyces cerevisiae was examined by density transfer experiments. After transfer to light medium, significant amounts of fully heavy dsRNA persisted over a number of cell doublings. In addition, very little material of hybrid density was ever formed, and the accumulation of fully light material began as early as 0.5 doubling after transfer to light medium. Our results are compatible with a conservative mode of replication or with a semiconservative mode of replication carried out by a small portion of the total dsRNA population. In additional experiments the synthesis of dsRNA relative to the cell cycle was studied. This was done by determining the ratio of short-term to long-term radioactive label in size-separated cell fractions of a prelabeled exponential culture. The ratio of short-term to long-term label remained constant for all fractions, implying that dsRNA is synthesized throughout the cell cycle, increasing through the cell cycle at an exponential rate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURDON R. H., BILLETER M. A., WEISSMANN C., WARNER R. C., OCHOA S., KNIGHT C. A. REPLICATION OF VIRAL RNA, V. PRESENCE OF A VIRUS-SPECIFIC DOUBLE-STRANDED RNA IN LEAVES INFECTED WITH TOBACCO MOSAIC VIRUS. Proc Natl Acad Sci U S A. 1964 Sep;52:768–775. doi: 10.1073/pnas.52.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastia D., Sueoka N. Studies on the late replication of phage lambda: rolling-circle replication of the wild type and a partially suppressed strain, Oam29 Pam80. J Mol Biol. 1975 Oct 25;98(2):305–320. doi: 10.1016/s0022-2836(75)80120-3. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Replication of a bacterial plasmid and an episome in Escherichia coli. Biochemistry. 1970 Jan 20;9(2):399–406. doi: 10.1021/bi00804a029. [DOI] [PubMed] [Google Scholar]
- Bevan E. A., Herring A. J., Mitchell D. J. Preliminary characterization of two species of dsRNA in yeast and their relationship to the "killer" character. Nature. 1973 Sep 14;245(5420):81–86. doi: 10.1038/245081b0. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. Mouse L cell mitochondrial DNA molecules are selected randomly for replication throughout the cell cycle. Cell. 1977 Aug;11(4):719–727. doi: 10.1016/0092-8674(77)90286-0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Buck K. W. Semi-conservative replication of double-stranded RNA by a virion-associated RNA polymerase. Biochem Biophys Res Commun. 1978 Oct 16;84(3):639–645. doi: 10.1016/0006-291x(78)90753-2. [DOI] [PubMed] [Google Scholar]
- Diaz-Ruiz J. R., Kaper J. M. Isolation of viral double-stranded RNAs using a LiCl fractionation procedure. Prep Biochem. 1978;8(1):1–17. doi: 10.1080/00327487808068215. [DOI] [PubMed] [Google Scholar]
- Elliott S. G., McLaughlin C. S. Regulation of RNA synthesis in yeast. III. Synthesis during the cell cycle. Mol Gen Genet. 1979 Feb 1;169(3):237–243. doi: 10.1007/BF00382269. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Gustafsson P., Nordström K., Perram J. W. Selection and timing of replication of plasmids R1drd-19 and F'lac in Escherichia coli. Plasmid. 1978 Feb;1(2):187–203. doi: 10.1016/0147-619x(78)90038-0. [DOI] [PubMed] [Google Scholar]
- Herring A. J., Bevan E. A. Virus-like particles associated with the double-stranded RNA species found in killer and sensitive strains of the yeast Saccharomyces cerevisiae. J Gen Virol. 1974 Mar;22(3):387–394. doi: 10.1099/0022-1317-22-3-387. [DOI] [PubMed] [Google Scholar]
- Herring A. J., Bevan E. A. Yeast virus-like particles possess a capsid-associated single-stranded RNA polymerase. Nature. 1977 Aug 4;268(5619):464–466. doi: 10.1038/268464a0. [DOI] [PubMed] [Google Scholar]
- Hollings M. Mycoviruses: viruses that infect fungi. Adv Virus Res. 1978;22:1–53. doi: 10.1016/s0065-3527(08)60771-x. [DOI] [PubMed] [Google Scholar]
- Holm C. A., Oliver S. G., Newman A. M., Holland L. E., McLaughlin C. S., Wagner E. K., Warner R. C. The molecular weight of yeast P1 double-stranded RNA. J Biol Chem. 1978 Nov 25;253(22):8332–8336. [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- KIRBY K. S. ISOLATION AND CHARACTERIZATION OF RIBOSOMAL RIBONUCLEIC ACID. Biochem J. 1965 Jul;96:266–269. doi: 10.1042/bj0960266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUDLUM D. B., WARNER R. C. EQUILIBRIUM CENTRIFUGATION IN CESIUM SULFATE SOLUTIONS. J Biol Chem. 1965 Jul;240:2961–2965. [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S. G., McCREADY S. J., Holm C., Sutherland P. A., McLaughlin C. S., Cox B. S. Biochemical and physiological studies of the yeast virus-like particle. J Bacteriol. 1977 Jun;130(3):1303–1309. doi: 10.1128/jb.130.3.1303-1309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S. G., McLaughlin C. S. The regulation of RNA synthesis in yeast. I: Starvation experiments. Mol Gen Genet. 1977 Jul 20;154(2):145–153. doi: 10.1007/BF00330830. [DOI] [PubMed] [Google Scholar]
- Ratti G., Buck K. W. Semi-conservative transcription in particles of a double-stranded RNA mycovirus. Nucleic Acids Res. 1978 Oct;5(10):3843–3854. doi: 10.1093/nar/5.10.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix J. D., Bird A., Barkken A. Ribosomal RNA gene amplification by rolling circles. J Mol Biol. 1974 Aug 15;87(3):473–487. doi: 10.1016/0022-2836(74)90098-9. [DOI] [PubMed] [Google Scholar]
- Rownd R. Replication of a bacterial episome under relaxed control. J Mol Biol. 1969 Sep 28;44(3):387–402. doi: 10.1016/0022-2836(69)90368-4. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Christman J. K., Acs G. The reovirus replicative cycle. Annu Rev Biochem. 1976;45:375–408. doi: 10.1146/annurev.bi.45.070176.002111. [DOI] [PubMed] [Google Scholar]
- Sweeney T. K., Tate A., Fink G. R. A study of the transmission and structure of double stranded RNAs associated with the killer phenomenon in Saccharomyces cerevisiae. Genetics. 1976 Sep;84(1):27–42. doi: 10.1093/genetics/84.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Burbank D. E., Cuppels D. A., Lane L. C., Vidaver A. K. Semiconservative synthesis of single-stranded RNA by bacteriophage phi 6 RNA polymerase. J Virol. 1980 Feb;33(2):769–773. doi: 10.1128/jvi.33.2.769-773.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M., Katterman F., Fink G. R. Yeast killer mutants with altered double-stranded ribonucleic acid. J Bacteriol. 1974 Feb;117(2):681–686. doi: 10.1128/jb.117.2.681-686.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J. D., Leibowitz M. J., Wickner R. B. Virion DNA-independent RNA polymerase from Saccharomyces cerevisiae. Nucleic Acids Res. 1980 Jun 11;8(11):2349–2363. doi: 10.1093/nar/8.11.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Chromosomal genes essential for replication of a double-stranded RNA plasmid of Saccharomyces cerevisiae: the killer character of yeast. J Mol Biol. 1976 Aug 15;105(3):427–443. doi: 10.1016/0022-2836(76)90102-9. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Fennell D. J. Apparent dispersive replication of yeast mitochondrial DNA as revealed by density labelling experiments. Mol Gen Genet. 1974;131(3):193–207. doi: 10.1007/BF00267959. [DOI] [PubMed] [Google Scholar]
- Zakian V. A., Brewer B. J., Fangman W. L. Replication of each copy of the yeast 2 micron DNA plasmid occurs during the S phase. Cell. 1979 Aug;17(4):923–934. doi: 10.1016/0092-8674(79)90332-5. [DOI] [PubMed] [Google Scholar]


