Abstract
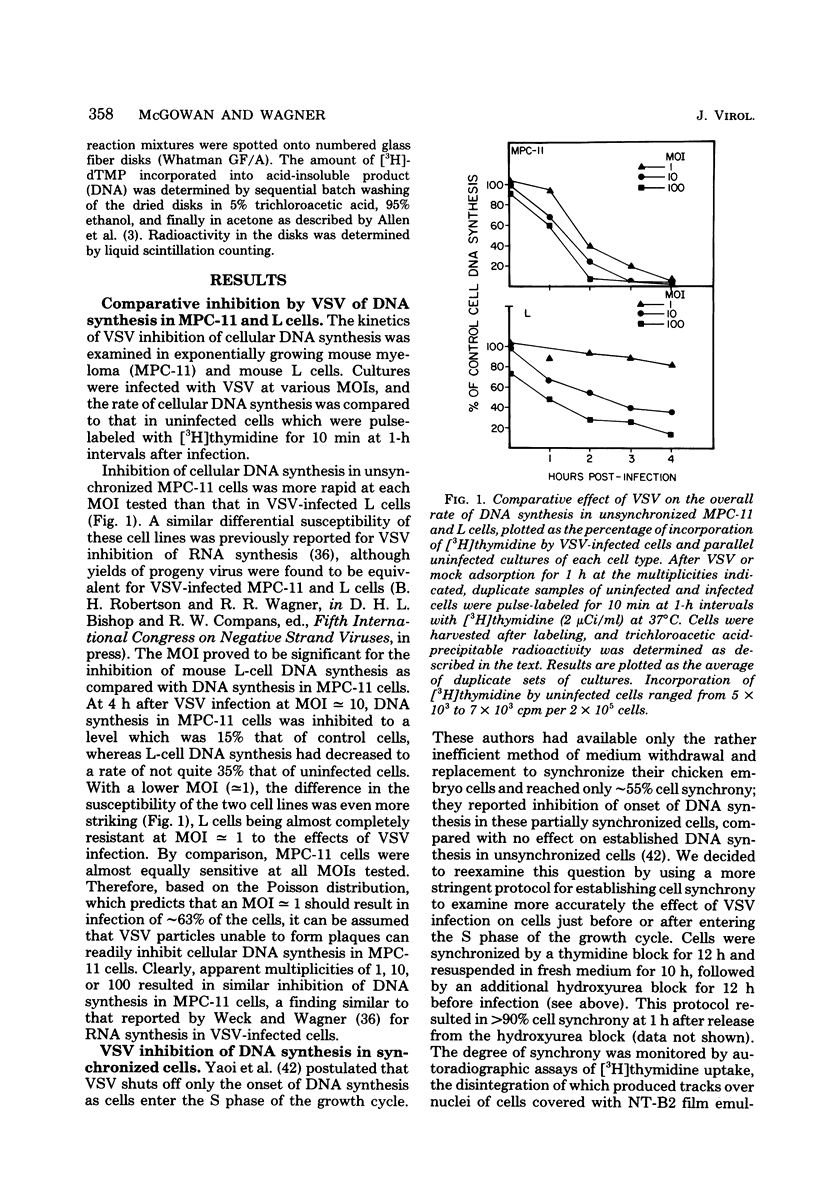
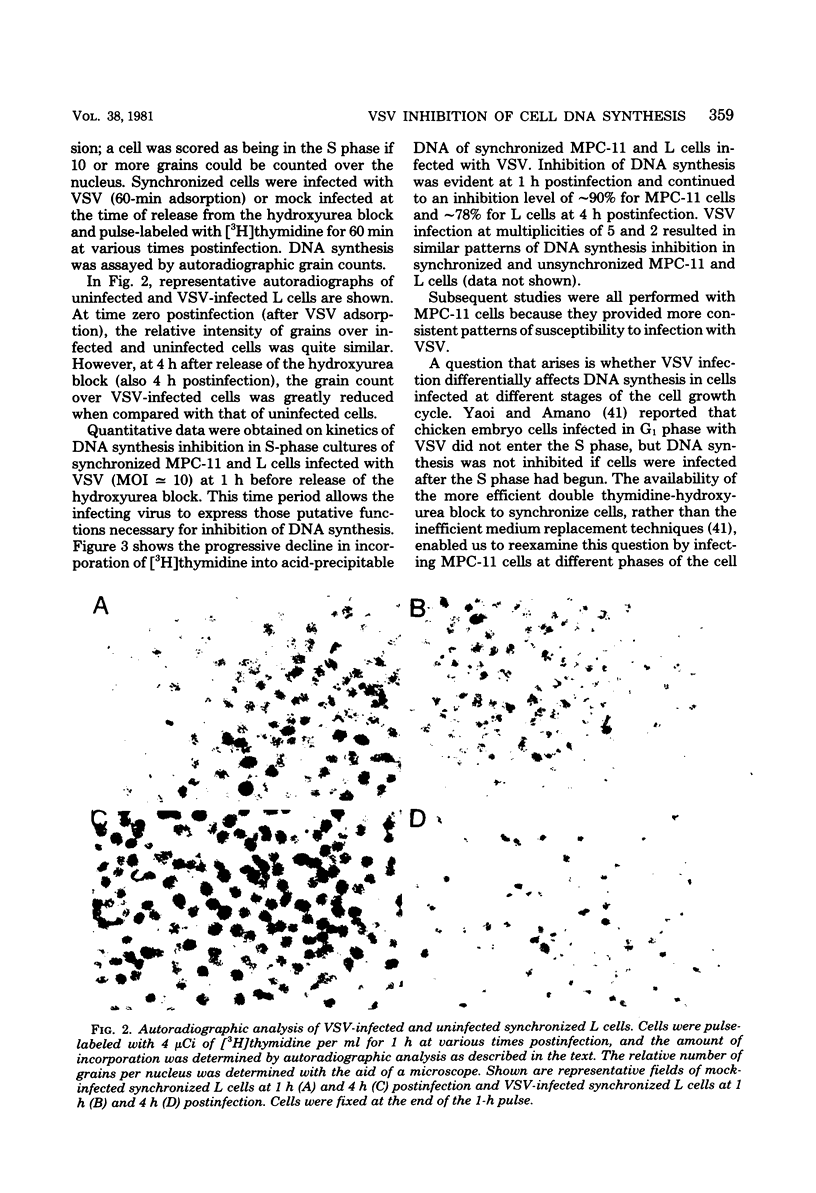
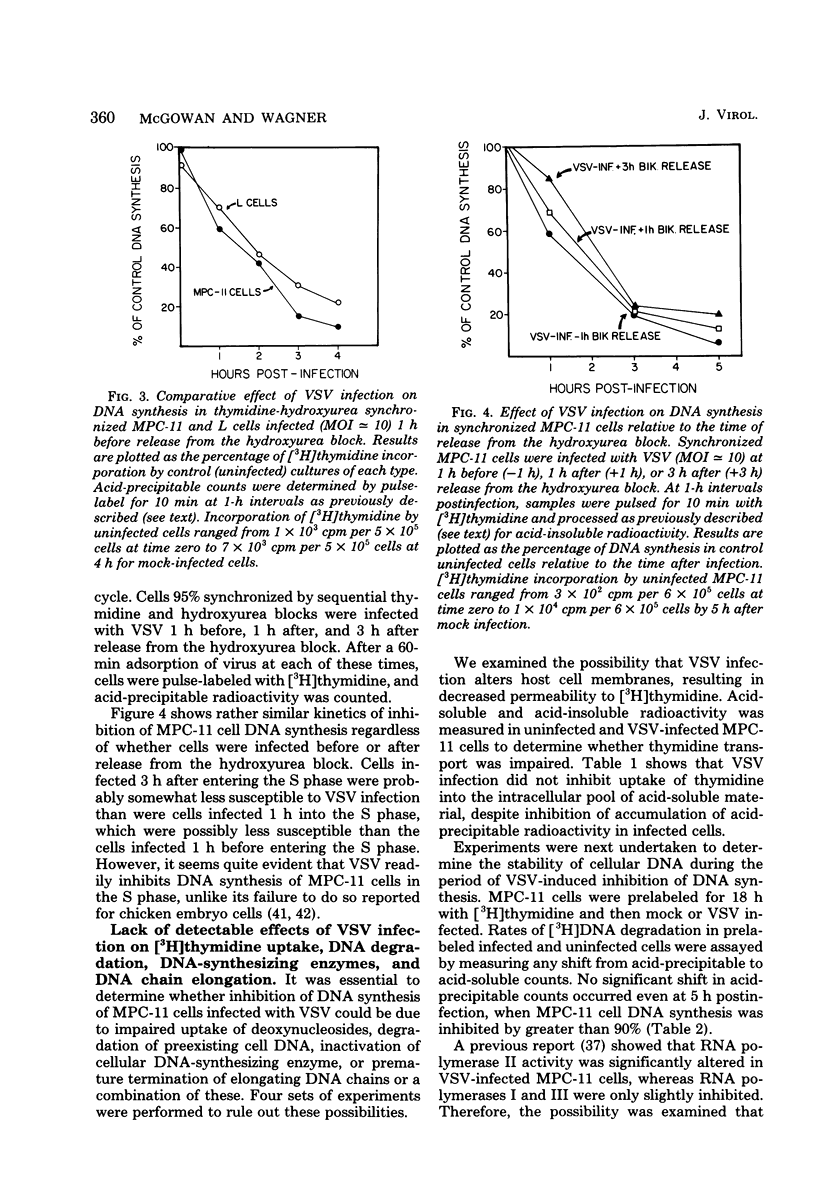
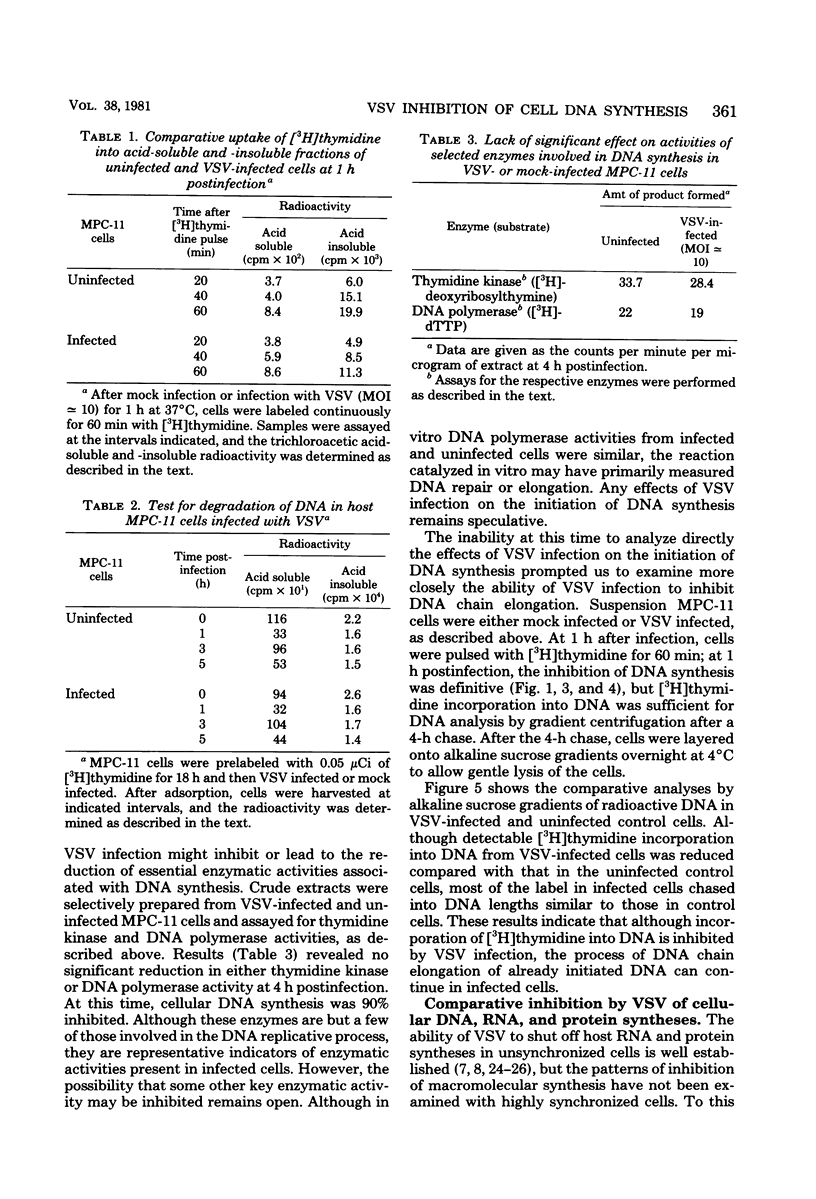
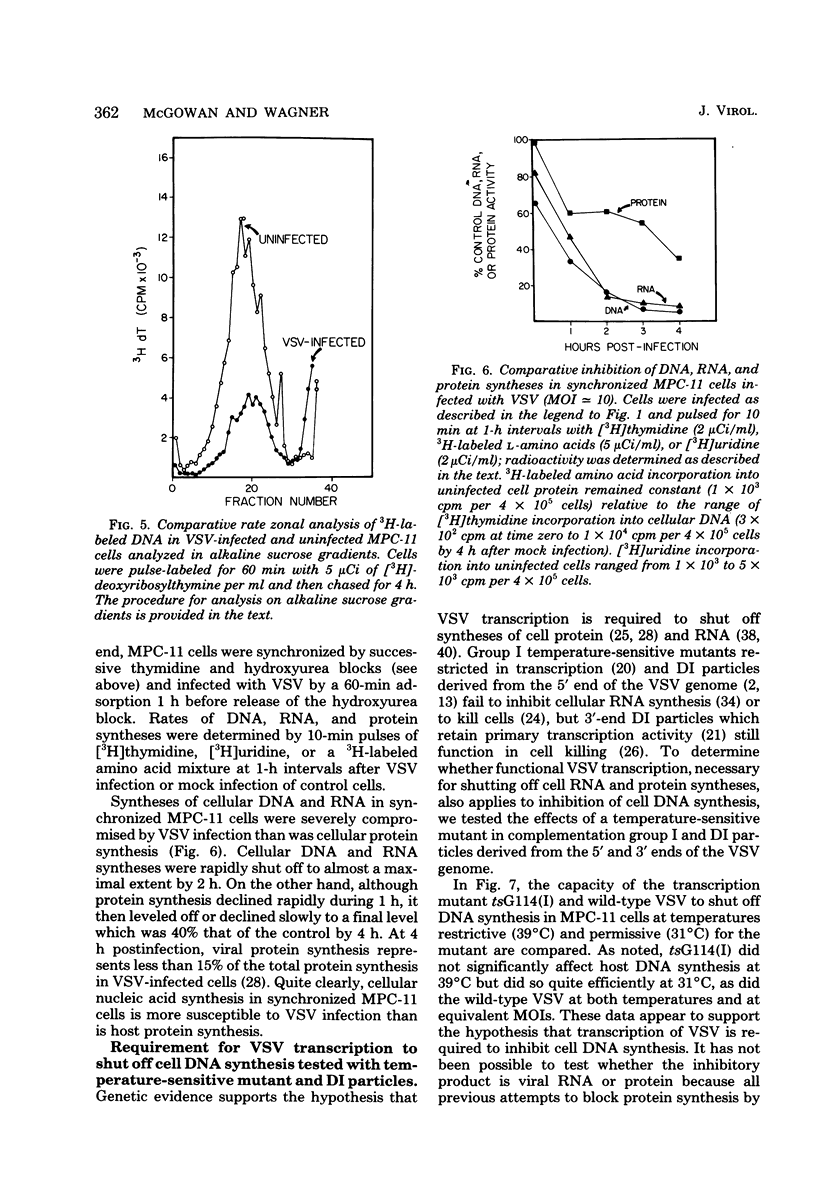
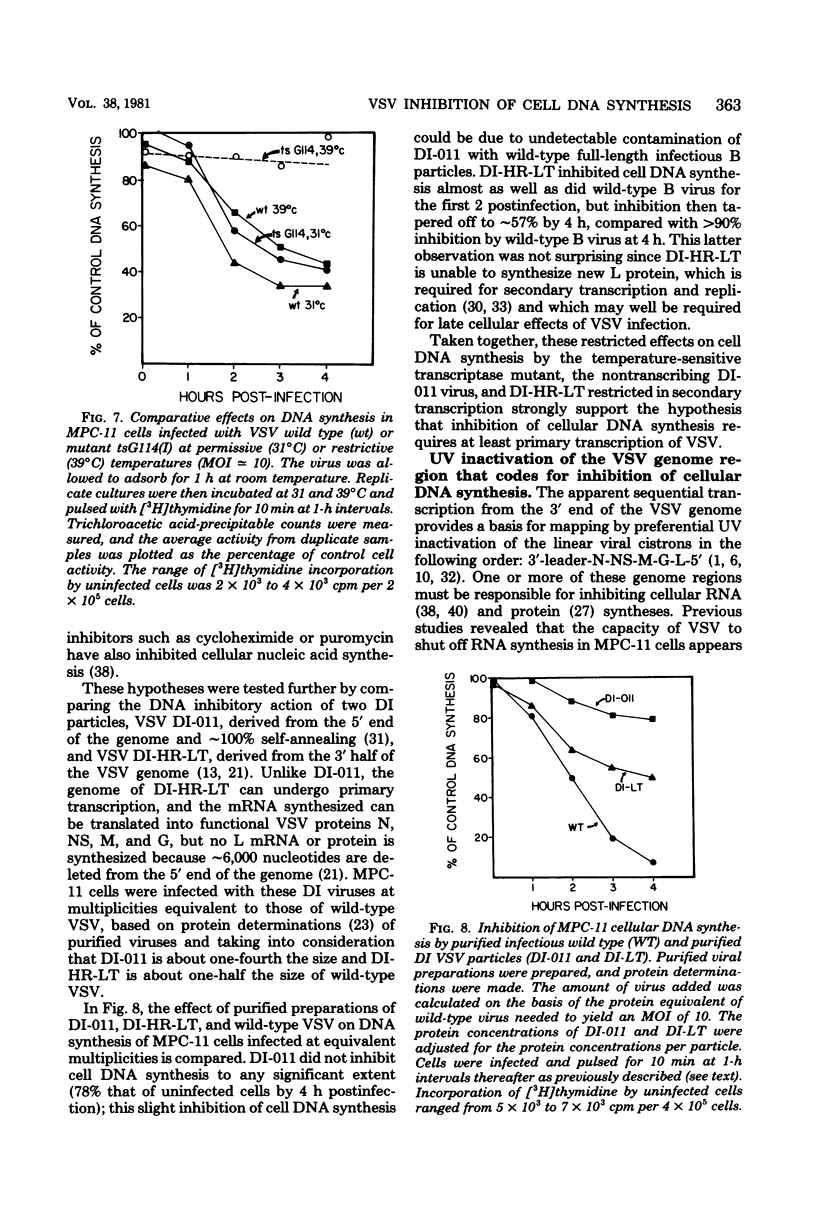
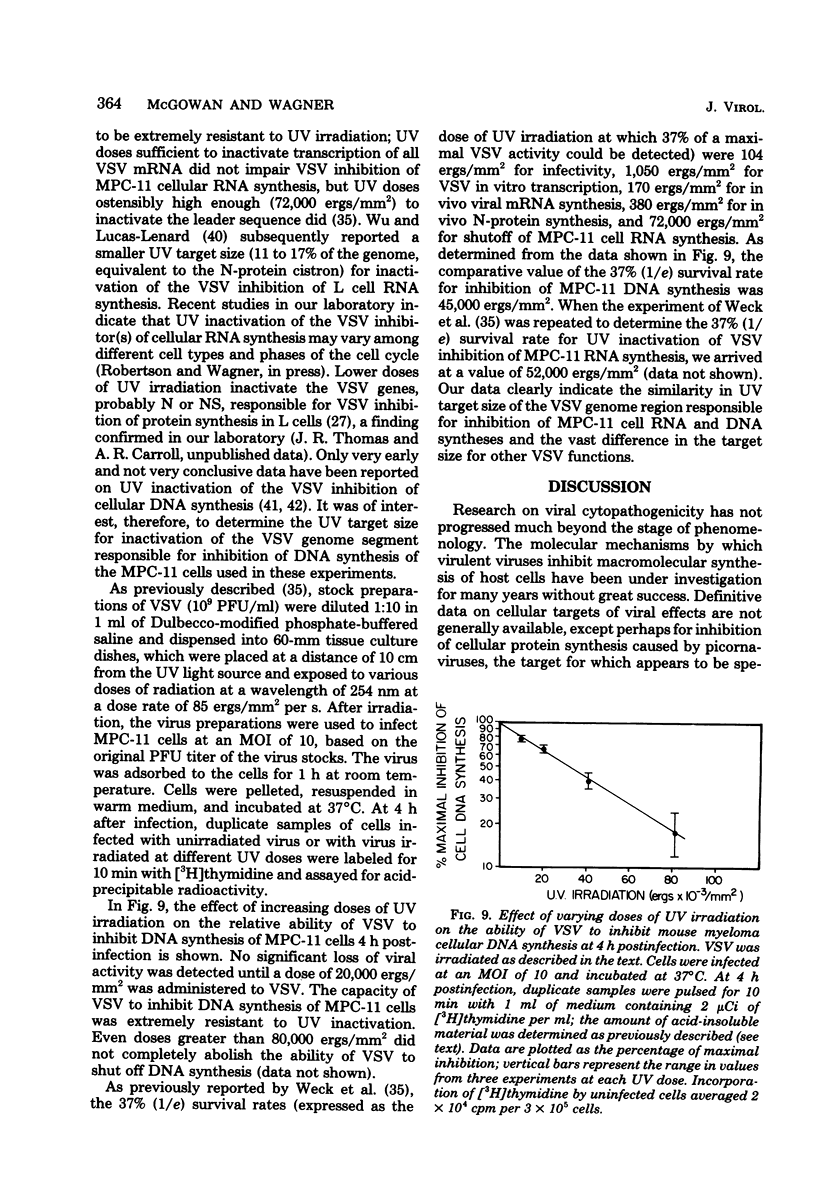
DNA synthesis in mouse myeloma (MPC-11) cells and L cells was rapidly and progressively inhibited by infection with vesicular stomatitis virus (VSV). No significant difference in cellular DNA synthesis inhibition was noted between synchronized and unsynchronized cells, nor did synchronized cells vary in their susceptibility to VSV infection after release from successive thymidine and hydroxyurea blocks. Cellular RNA synthesis was inhibited to about the same extent as DNA synthesis, but cellular protein synthesis was less affected by VSV at the same multiplicity of infection. The effect of VSV on cellular DNA synthesis could not be attributed to degradation of existing DNA or to decreased uptake of deoxynucleoside triphosphates, nor were DNA polymerase and thymidine kinase activities significantly different in VSV-infected and uninfected cell extracts. Analysis by alkaline sucrose gradients of DNA in pulse-labeled uninfected and VSV-infected cells indicated that VSV infection did not appear to influence DNA chain elongation. Cellular DNA synthesis was not significantly inhibited by infection with the VSV polymerase mutant tsG114(I) at the restrictive temperature or by infection with defective-interfering VSV DI-011 (5' end of the genome), but DI-HR-LT (3' end of genome) exhibited initially rapid but not prolonged inhibition of MPC-11 cell DNA synthesis. DNA synthesis inhibitory activity of wild-type VSV was only slowly and partially inactivated by very large doses of UV irradiation. These data suggest that, as in the effect of VSV on cellular RNA synthesis (Weck et al., J. Virol. 30:746-753, 1979), inhibition of cellular DNA synthesis by VSV requires transcription of a small segment of the viral genome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi T., Lazzarini R. A. Elementary aspects of autointerference and the replication of defective interfering virus particles. Virology. 1978 Jun 1;87(1):152–163. doi: 10.1016/0042-6822(78)90167-8. [DOI] [PubMed] [Google Scholar]
- Allen G. P., O'Callaghan D. J., Randall C. C. Purification and characterization of equine herpesvirus-induced DNA. Virology. 1977 Jan;76(1):395–408. doi: 10.1016/0042-6822(77)90311-7. [DOI] [PubMed] [Google Scholar]
- Apriletti J. W., Penhoet E. E. Cellular RNA synthesis in normal and mengovirus-infected L-929 cells. J Biol Chem. 1978 Jan 25;253(2):603–611. [PubMed] [Google Scholar]
- Atkins G. J. The effect of infection with Sindbis virus and its temperature-sensitive mutants on cellular protein and DNA synthesis. Virology. 1976 Jun;71(2):593–597. doi: 10.1016/0042-6822(76)90384-6. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxt B., Bablanian R. Mechansims of vesicular stomatitis virus-induced cytopathic effects. II. Inhibition of macromolecular synthesis induced by infectious and defective-interfering particles. Virology. 1976 Jul 15;72(2):383–392. doi: 10.1016/0042-6822(76)90167-7. [DOI] [PubMed] [Google Scholar]
- Carrasco L. The inhibition of cell functions after viral infection. A proposed general mechanism. FEBS Lett. 1977 Apr 1;76(1):11–15. doi: 10.1016/0014-5793(77)80110-5. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Adenosine-5'-O-(3-thiotriphosphate) as an affinity probe for studying leader RNA's transcribed by vesicular stomatitis virus. J Biol Chem. 1979 Oct 10;254(19):9339–9341. [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Mapping and initiation studies on the leader RNA of vesicular stomatitis virus. Virology. 1977 Mar;77(1):260–268. doi: 10.1016/0042-6822(77)90423-8. [DOI] [PubMed] [Google Scholar]
- Ensminger W. D., Tamm I. Inhibition of synchronized cellular deoxyribonucleic acid synthesis during Newcastle disease virus, mengovirus, or reovirus infection. J Virol. 1970 Jun;5(6):672–676. doi: 10.1128/jvi.5.6.672-676.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensminger W. D., Tamm I. The step in cellular DNA synthesis blocked by Newcastle disease or mengovirus infection. Virology. 1970 Jan;40(1):152–165. doi: 10.1016/0042-6822(70)90387-9. [DOI] [PubMed] [Google Scholar]
- Epstein D. A., Herman R. C., Chien I., Lazzarini R. A. Defective interfering particle generated by internal deletion of the vesicular stomatitis virus genome. J Virol. 1980 Feb;33(2):818–829. doi: 10.1128/jvi.33.2.818-829.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand A., Bishop D. H. Primary in vivo transcription of vesicular stomatitis virus and temperature-sensitive mutants of five vesicular stomatitis virus complementation groups. J Virol. 1973 Dec;12(6):1238–1252. doi: 10.1128/jvi.12.6.1238-1252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty N. Analysis of uridine incorporation in chicken embryo cells infected by vesicular stomatitis virus and its temperature-sensitive mutants: uridine transport. J Virol. 1975 Jan;15(1):8–15. doi: 10.1128/jvi.15.1.8-15.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., PETERSON J. A. NUCLEIC ACID AND PROTEIN SYNTHESIS DURING POLIOVIRUS INFECTION OF HUMAN CELLS. J Mol Biol. 1964 Apr;8:556–575. doi: 10.1016/s0022-2836(64)80011-5. [DOI] [PubMed] [Google Scholar]
- Hand R., Ensminger W. D., Tamm I. Cellular DNA replication in infections with cytocidal RNA viruses. Virology. 1971 Jun;44(3):527–536. doi: 10.1016/0042-6822(71)90366-7. [DOI] [PubMed] [Google Scholar]
- Helentjaris T., Ehrenfeld E., Brown-Luedi M. L., Hershey J. W. Alterations in initiation factor activity from poliovirus-infected HeLa cells. J Biol Chem. 1979 Nov 10;254(21):10973–10978. [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Inhibition of cellular RNA synthesis by nonreplicating vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1579–1584. doi: 10.1073/pnas.54.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. M., Wagner R. R. Location of the transcription defect in group I temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1974 Jan;13(1):28–35. doi: 10.1128/jvi.13.1.28-35.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S., Simizu B., Hashimoto K., Oya A., Yamada M. Inhibition of DNA synthesis in BHK cells infected with western equine encephalitis virus. 1. Induction of an inhibitory factor of cellular DNA polymerase activity. Virology. 1979 Apr 30;94(2):314–322. doi: 10.1016/0042-6822(79)90464-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Cell killing by viruses. I. Comparison of cell-killing, plaque-forming, and defective-interfering particles of vesicular stomatitis virus. Virology. 1974 Feb;57(2):321–338. doi: 10.1016/0042-6822(74)90172-x. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Cell killing by viruses. II. Cell killing by vesicular stomatitis virus: a requirement for virion-derived transcription. Virology. 1975 Jan;63(1):176–190. doi: 10.1016/0042-6822(75)90383-9. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J., Johnson L. D., Lazzarini R. A. Cell killing by viruses. V. Transcribing defective interfering particles of vesicular stomatitis virus function as cell-killing particles. Virology. 1977 Oct 1;82(1):242–246. doi: 10.1016/0042-6822(77)90048-4. [DOI] [PubMed] [Google Scholar]
- Marvaldi J., Sekellick M. J., Marcus P. I., Lucas-Lenard J. Inhibition of mouse L cell protein synthesis by ultraviolet-irradiated vesicular stomatitis virus requires viral transcription. Virology. 1978 Jan;84(1):127–133. doi: 10.1016/0042-6822(78)90224-6. [DOI] [PubMed] [Google Scholar]
- McAllister P. E., Wagner R. R. Differential inhibition of host protein synthesis in L cells infected with RNA - temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1976 May;18(2):550–558. doi: 10.1128/jvi.18.2.550-558.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. J., Allen G. P., Barnett J. M., Gentry G. A. Biochemical characterization of equine herpesvirus type 3-induced deoxythymidine kinase purified from lytically infected horse embryo dermal fibroblasts. J Virol. 1980 May;34(2):474–483. doi: 10.1128/jvi.34.2.474-483.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon M. G., Emerson S. U. Rebinding of transcriptase components (L and NS proteins) to the nucleocapsid template of vesicular stomatitis virus. J Virol. 1978 Sep;27(3):560–567. doi: 10.1128/jvi.27.3.560-567.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Leavitt R. W. Characterization of snap-back RNAs in vesicular stomatitis defective interfering virus particles. J Gen Virol. 1978 Jan;38(1):21–34. doi: 10.1099/0022-1317-38-1-21. [DOI] [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. Unique mode of transcription in vitro by Vesicular stomatitis virus. Cell. 1980 Aug;21(1):267–275. doi: 10.1016/0092-8674(80)90134-8. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Huang A. S. Inhibition of RNA and interferon synthesis in Krebs-2 cells infected with vesicular stomatitis virus. Virology. 1966 Jan;28(1):1–10. doi: 10.1016/0042-6822(66)90300-x. [DOI] [PubMed] [Google Scholar]
- Weck P. K., Carroll A. R., Shattuck D. M., Wagner R. R. Use of UV irradiation to identify the genetic information of vesicular stomatitis virus responsible for shutting off cellular RNA synthesis. J Virol. 1979 Jun;30(3):746–753. doi: 10.1128/jvi.30.3.746-753.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Wagner R. R. Inhibition of RNA synthesis in mouse myeloma cells infected with vesicular stomatitis virus. J Virol. 1978 Mar;25(3):770–780. doi: 10.1128/jvi.25.3.770-780.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Wagner R. R. Transcription of vesicular stomatitis virus is required to shut off cellular RNA synthesis. J Virol. 1979 Apr;30(1):410–413. doi: 10.1128/jvi.30.1.410-413.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Wagner R. R. Vesicular stomatitis virus infection reduces the number of active DNA-dependent RNA polymerases in myeloma cells. J Biol Chem. 1979 Jun 25;254(12):5430–5434. [PubMed] [Google Scholar]
- Wertz G. W., Youngner J. S. Inhibition of protein synthesis in L cells infected with vesicular stomatitis virus. J Virol. 1972 Jan;9(1):85–89. doi: 10.1128/jvi.9.1.85-89.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. S., Lucas-Lenard J. M. Inhibition of ribonucleic acid accumulation in mouse L cells infected with vesicular stomatitis virus requires viral ribonucleic acid transcription. Biochemistry. 1980 Feb 19;19(4):804–810. doi: 10.1021/bi00545a029. [DOI] [PubMed] [Google Scholar]
- Yaoi Y., Amano M. Inhibitory effect of ultraviolet-inactivated vesicular stomatitis virus on initiation o DNA synthesis in cultured chick embryo cells. J Gen Virol. 1970 Oct;9(1):69–75. doi: 10.1099/0022-1317-9-1-69. [DOI] [PubMed] [Google Scholar]
- Yaoi Y., Mitsui H., Amano M. Effect of U.v.-irradiated vesicular stomatitis virus on nucleic acid synthesis in chick embryo cells. J Gen Virol. 1970 Sep;8(3):165–172. doi: 10.1099/0022-1317-8-3-165. [DOI] [PubMed] [Google Scholar]



