Abstract
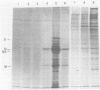
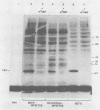
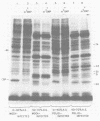
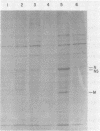
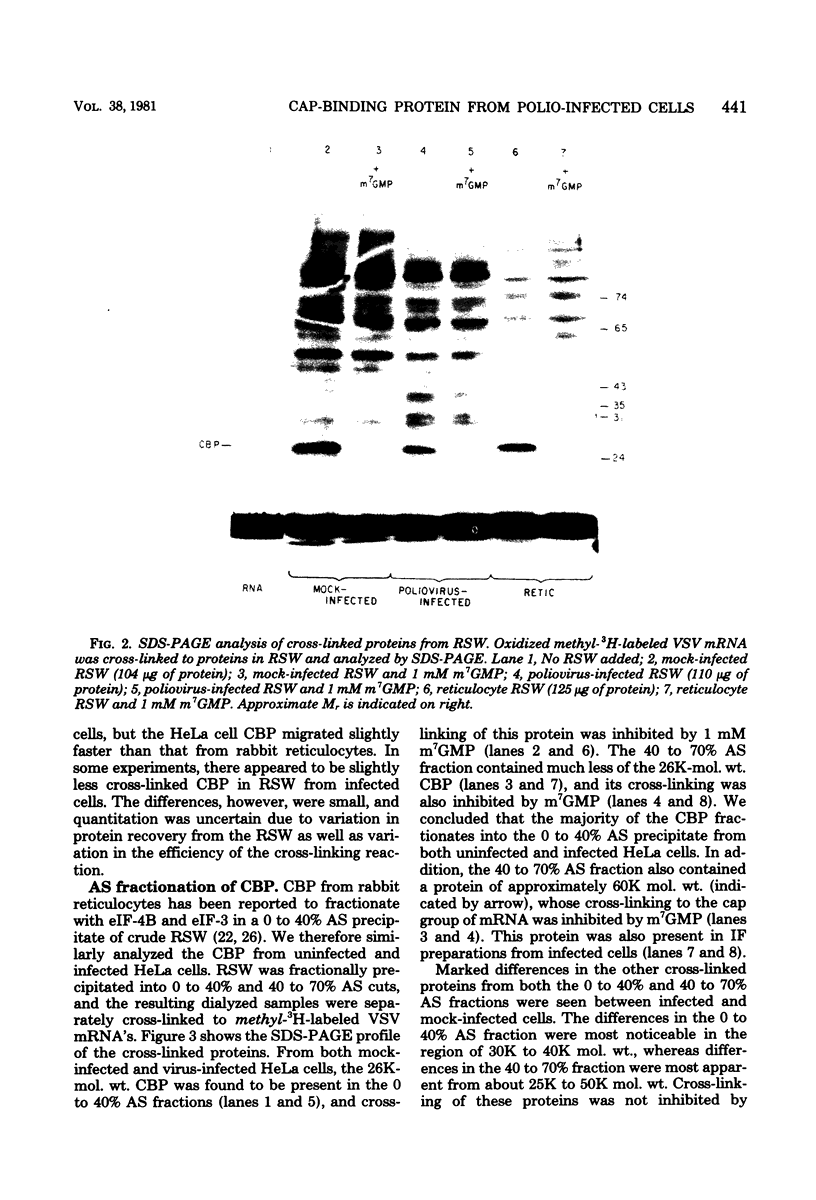
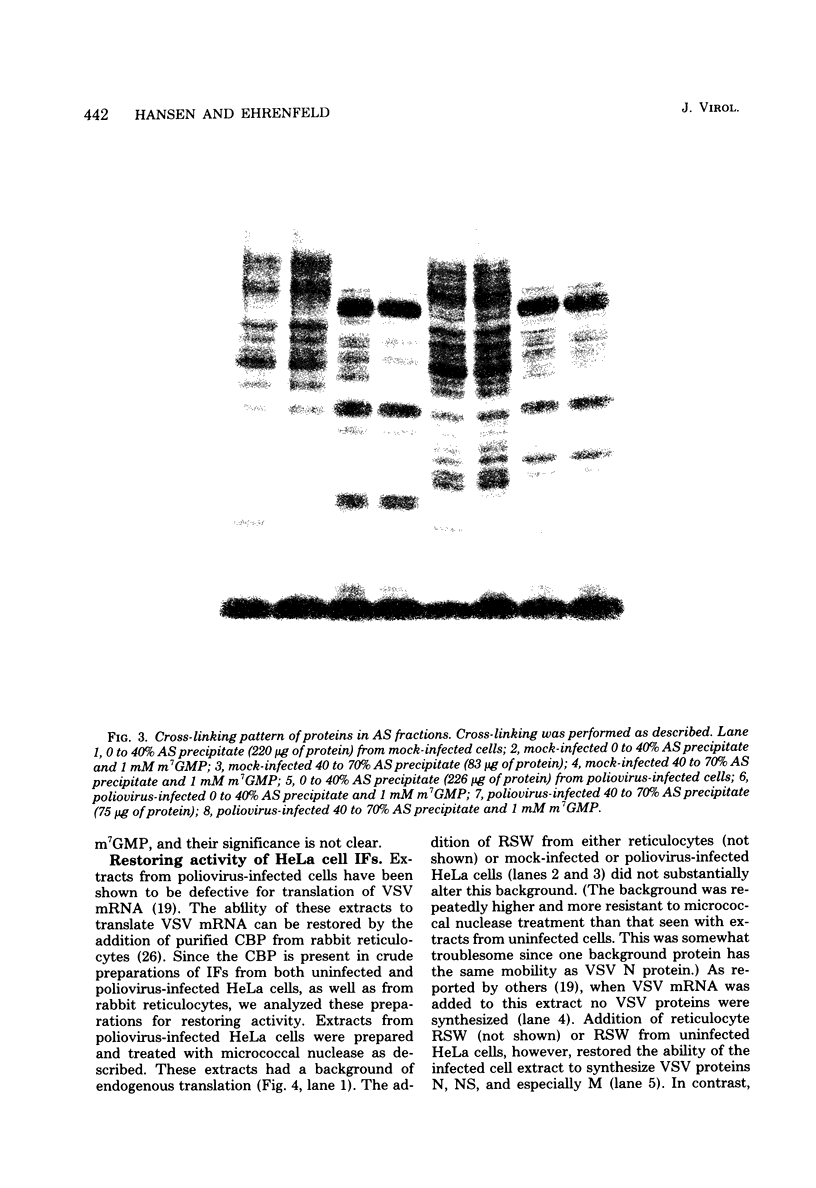
Crude preparations of initiation factors from mock-infected and poliovirus-infected HeLa cells were analyzed for the presence of proteins which could be cross-linked to the 5' cap group of mRNA. A protein having an apparent molecular weight of 26,000, similar to the cap-binding protein in rabbit reticulocytes described by Sonenberg and Shatkin (Proc. Natl. Acad. Sci. U.S.A. 75:4843-4847, 1978), was found in the ribosomal salt wash from both uninfected and infected cells. Cross-linking of this polypeptide was inhibited by the cap analog m7GMP. In addition, cross-linking of a protein having an approximate molecular weight of 60,000 was similarly inhibited by cap analog. The smaller cap-binding protein fractionated in a 0 to 40% ammonium sulfate precipitate of ribosomal salt wash; the larger protein was found in the 40 to 70% ammonium sulfate fraction. Although the cap-binding proteins were present in both mock-infected and poliovirus-infected ribosomal salt wash, only preparations from uninfected HeLa cells were able to restore translation of capped vesicular stomatitis virus mRNA by extracts prepared from poliovirus-infected cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K. 5'-terminal cap structure in eucaryotic messenger ribonucleic acids. Microbiol Rev. 1980 Jun;44(2):175–205. doi: 10.1128/mr.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt-Humphries S., Simonsen C., Ehrenfeld E. Full-length viral RNA synthesized in vitro by vesicular stomatitis virus-infected HeLa cell extracts. Virology. 1979 Jul 15;96(1):88–99. doi: 10.1016/0042-6822(79)90175-2. [DOI] [PubMed] [Google Scholar]
- Bergmann J. E., Trachsel H., Sonenberg N., Shatkin A. J., Lodish H. F. Characterization of rabbit reticulocyte factor(s) that stimulates the translation of mRNAs lacking 5'-terminal 7-methylguanosine. J Biol Chem. 1979 Mar 10;254(5):1440–1443. [PubMed] [Google Scholar]
- Brown B. A., Ehrenfeld E. Initiation factor preparations from poliovirus-infected cells restrict translation in reticulocyte lysates. Virology. 1980 Jun;103(2):327–339. doi: 10.1016/0042-6822(80)90191-9. [DOI] [PubMed] [Google Scholar]
- Brown B. A., Ehrenfeld E. Translation of poliovirus RNA in vitro: changes in cleavage pattern and initiation sites by ribosomal salt wash. Virology. 1979 Sep;97(2):396–405. doi: 10.1016/0042-6822(79)90350-7. [DOI] [PubMed] [Google Scholar]
- Brown D., Hansen J., Ehrenfeld E. Specificity of initiation factor preparations from poliovirus-infected cells. J Virol. 1980 May;34(2):573–575. doi: 10.1128/jvi.34.2.573-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Doyle M., Holland J. J. Virus-induced interference in heterologously infected HeLa cells. J Virol. 1972 Jan;9(1):22–28. doi: 10.1128/jvi.9.1.22-28.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E., Lund H. Untranslated vesicular stomatitis virus messenger RNA after poliovirus infection. Virology. 1977 Jul 15;80(2):297–308. doi: 10.1016/s0042-6822(77)80006-8. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E., Manis S. Inhibition of 80S initiation complex formation by infection with poliovirus. J Gen Virol. 1979 May;43(2):441–445. doi: 10.1099/0022-1317-43-2-441. [DOI] [PubMed] [Google Scholar]
- Helentjaris T., Ehrenfeld E., Brown-Luedi M. L., Hershey J. W. Alterations in initiation factor activity from poliovirus-infected HeLa cells. J Biol Chem. 1979 Nov 10;254(21):10973–10978. [PubMed] [Google Scholar]
- Helentjaris T., Ehrenfeld E. Control of protein synthesis in extracts from poliovirus-infected cells. I. mRNA discrimination by crude initiation factors. J Virol. 1978 May;26(2):510–521. doi: 10.1128/jvi.26.2.510-521.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen G., Detjen B. M., Thach R. E. Shutoff of HeLa cell protein synthesis by encephalomyocarditis virus and poliovirus: a comparative study. J Virol. 1980 Jul;35(1):150–156. doi: 10.1128/jvi.35.1.150-156.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann Y., Goldstein E., Penman S. Poliovirus-induced inhibition of polypeptide initiation in vitro on native polyribosomes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1834–1838. doi: 10.1073/pnas.73.6.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz R., Penman S. Regulation of protein synthesis in HeLa cells. 3. Inhibition during poliovirus infection. J Virol. 1971 Nov;8(5):661–668. doi: 10.1128/jvi.8.5.661-668.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukrishnan S., Morgan M., Banerjee A. K., Shatkin A. J. Influence of 5'-terminal m7G and 2'--O-methylated residues on messenger ribonucleic acid binding to ribosomes. Biochemistry. 1976 Dec 28;15(26):5761–5768. doi: 10.1021/bi00671a012. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Trachsel H., Leong K., Baltimore D. Inhibition of translation by poliovirus: inactivation of a specific initiation factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2732–2736. doi: 10.1073/pnas.75.6.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Rupprecht K. M., Hecht S. M., Shatkin A. J. Eukaryotic mRNA cap binding protein: purification by affinity chromatography on sepharose-coupled m7GDP. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4345–4349. doi: 10.1073/pnas.76.9.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Shatkin A. J. Reovirus mRNA can be covalently crosslinked via the 5' cap to proteins in initiation complexes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4288–4292. doi: 10.1073/pnas.74.10.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundkvist I. C., Staehelin T. Structure and function of free 40 S ribosome subunits: Characterization of initiation factors. J Mol Biol. 1975 Dec 15;99(3):401–418. doi: 10.1016/s0022-2836(75)80135-5. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Sonenberg N., Shatkin A. J., Rose J. K., Leong K., Bergmann J. E., Gordon J., Baltimore D. Purification of a factor that restores translation of vesicular stomatitis virus mRNA in extracts from poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):770–774. doi: 10.1073/pnas.77.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]