Abstract
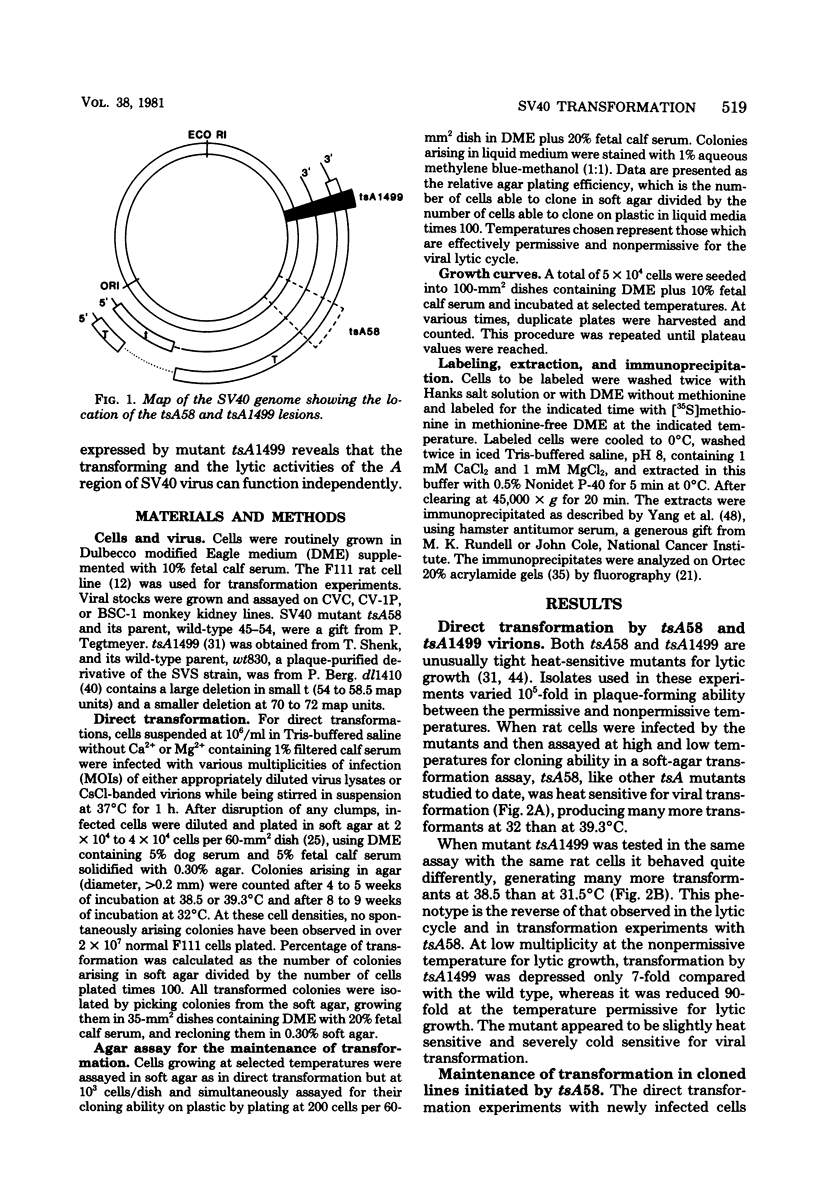
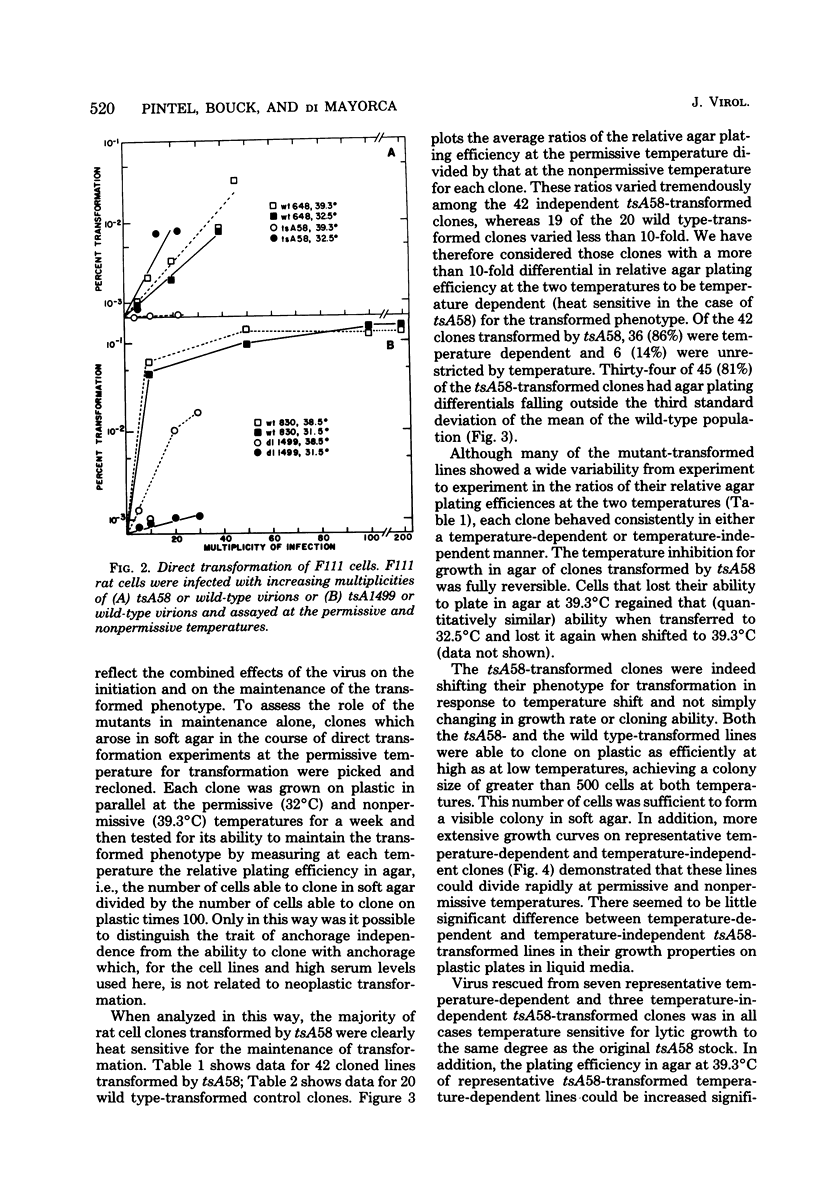
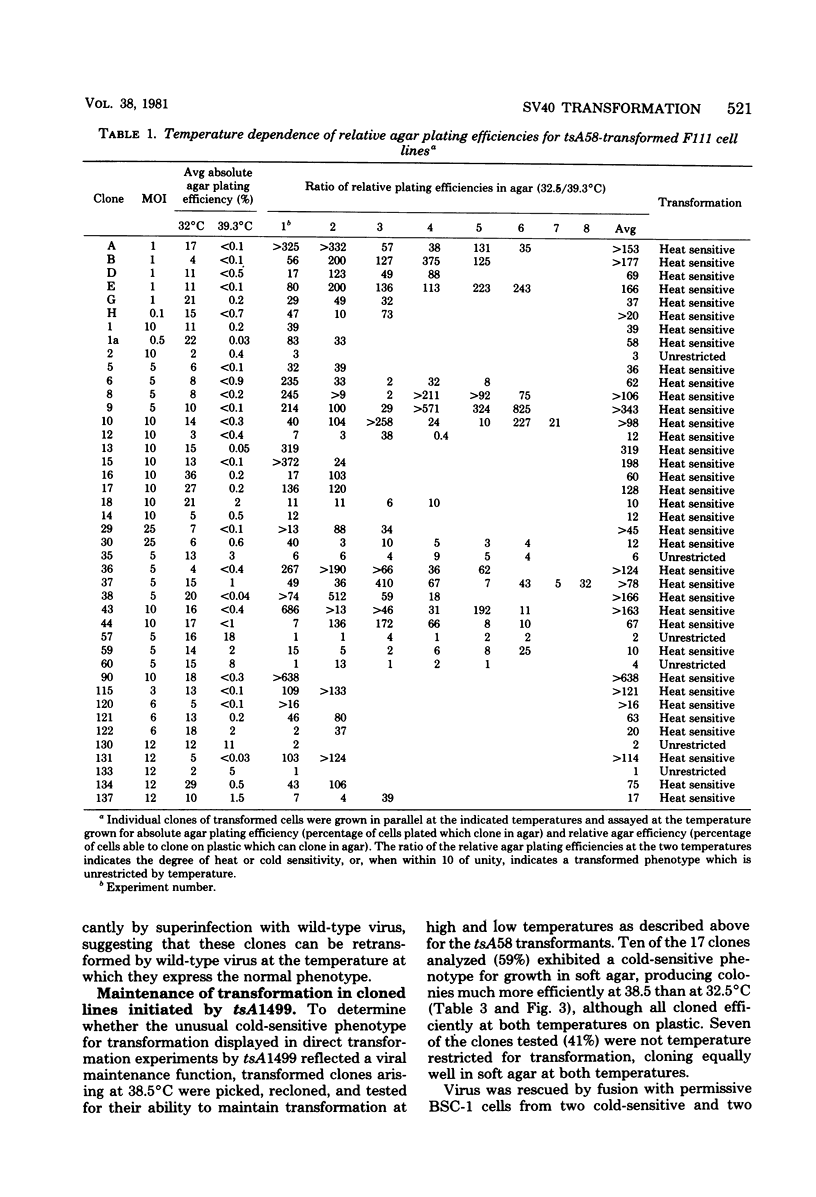
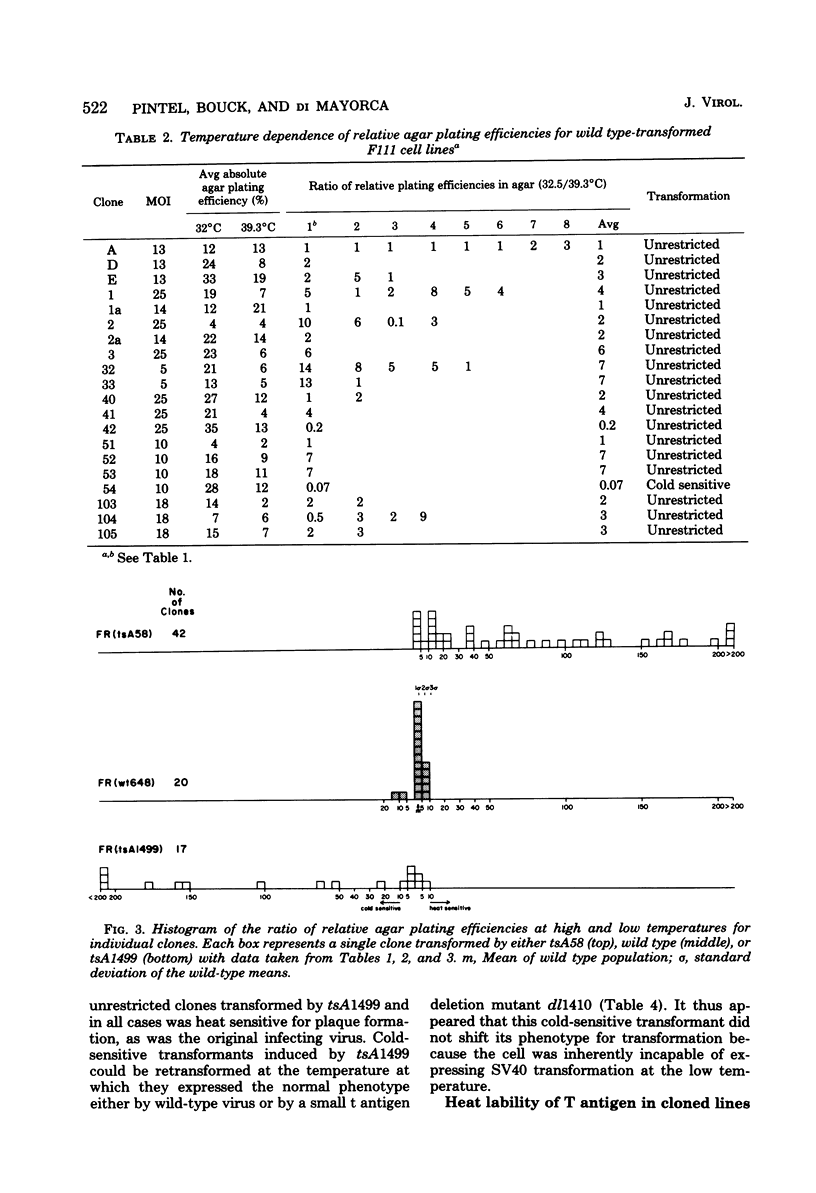
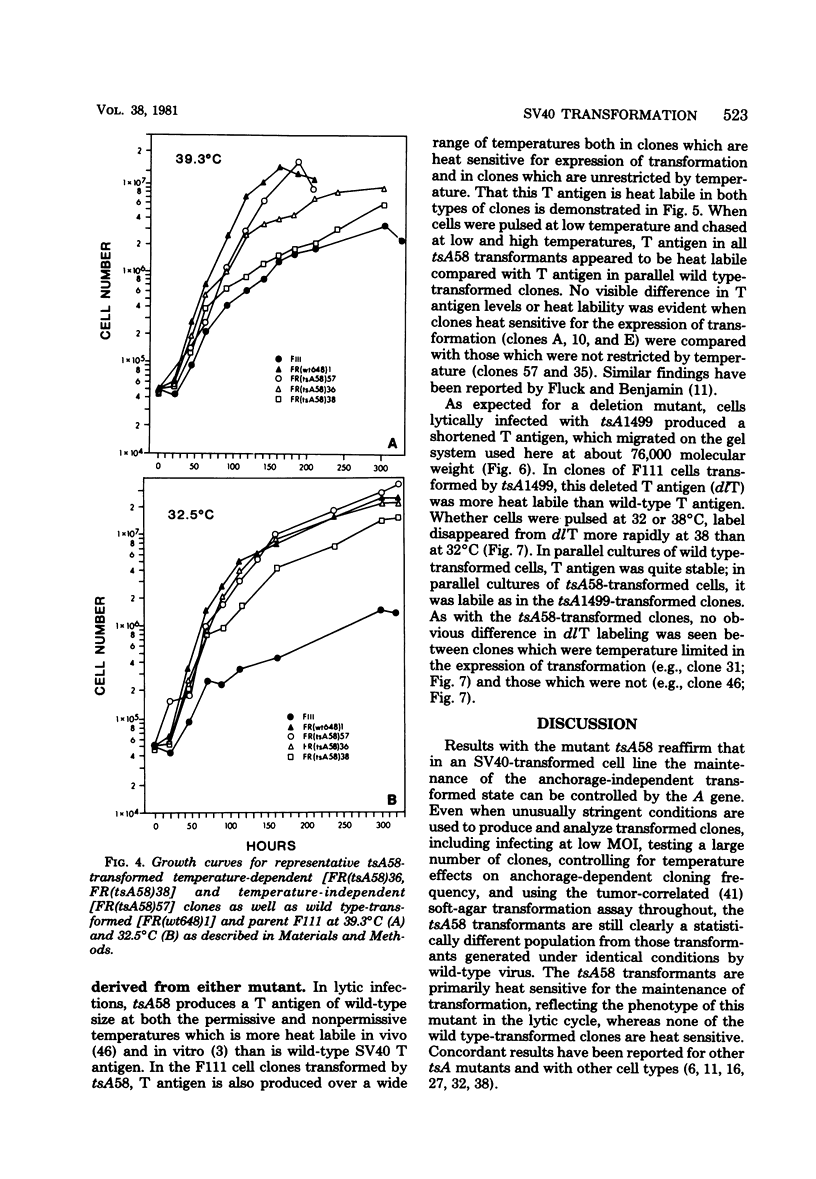
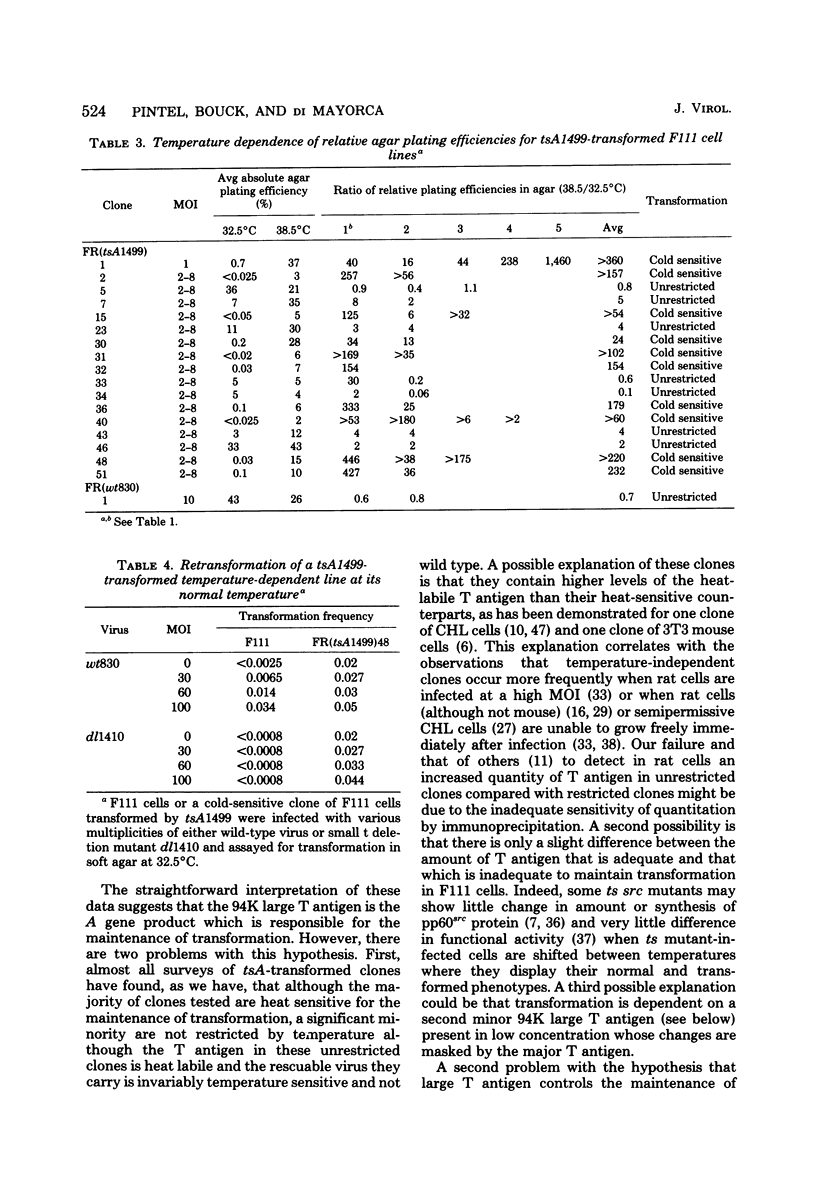
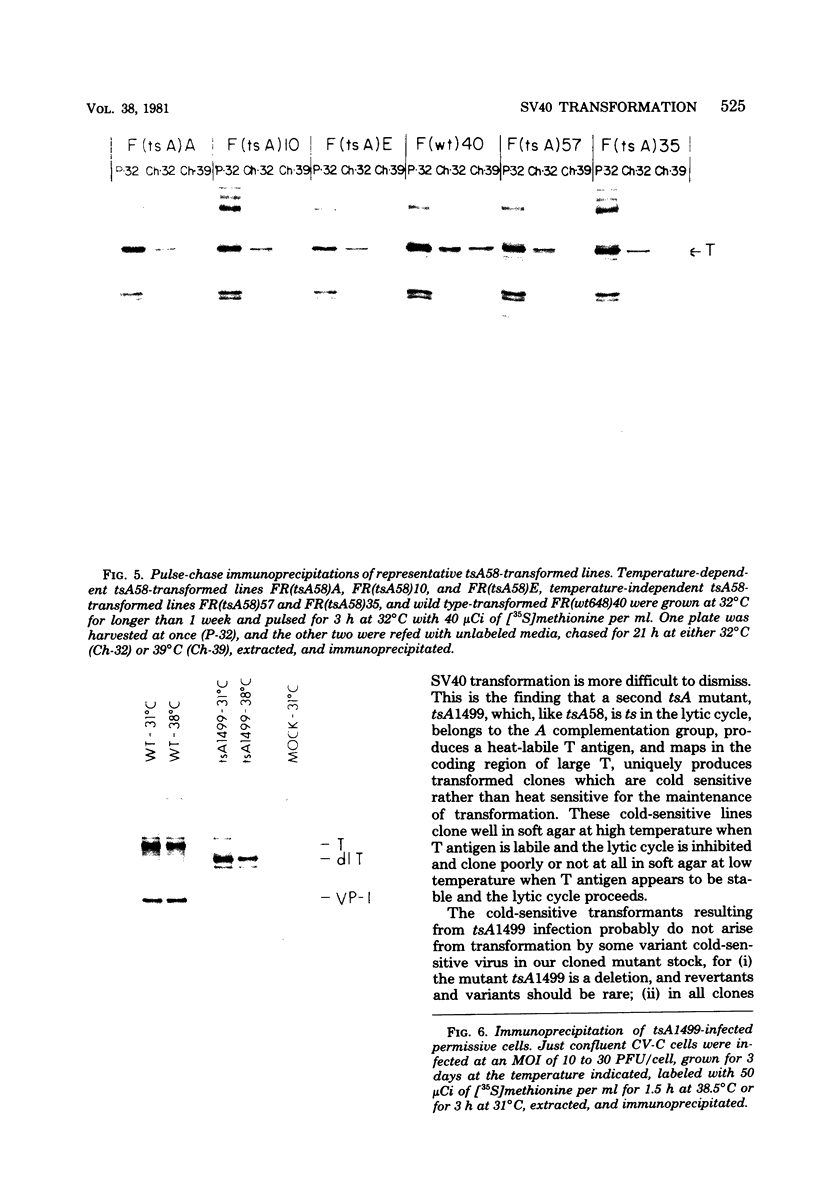
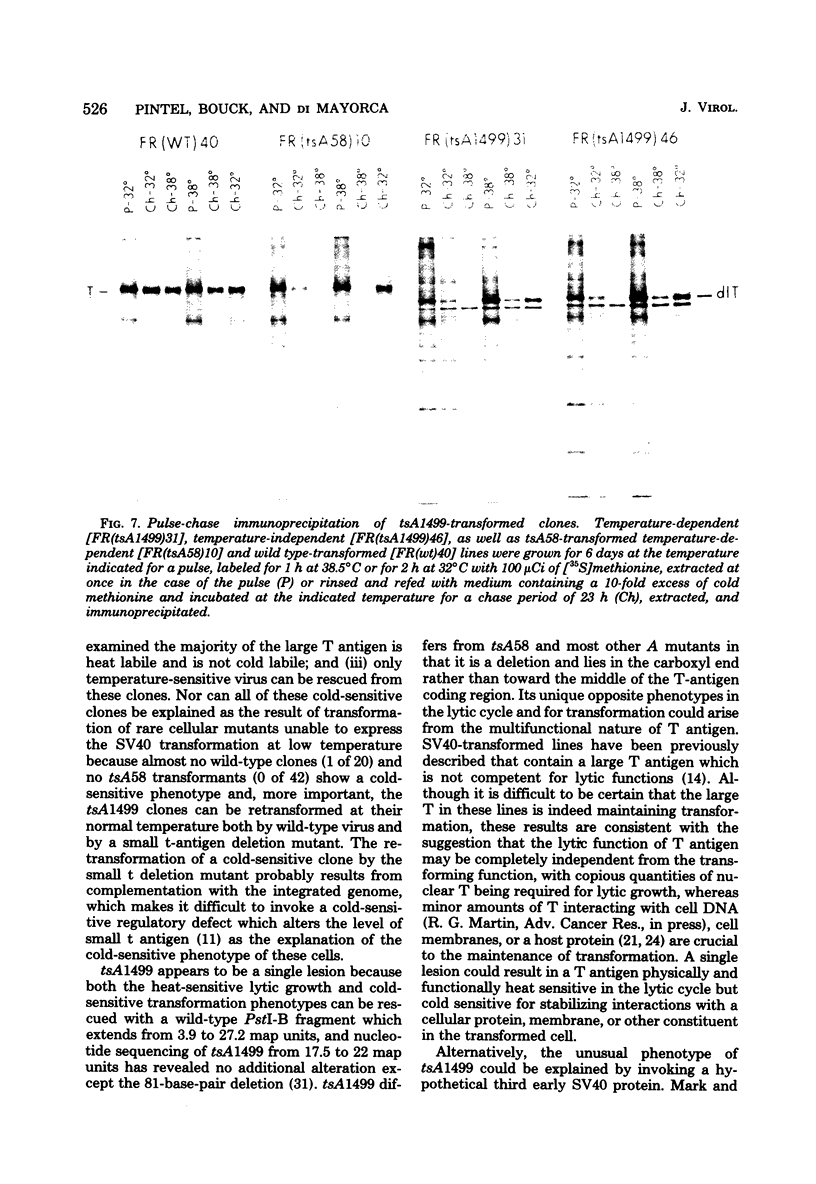
Thirty-six of 40 rat cell clones transformed to anchorage independence at low multiplicity of infection by simian virus 40 tsA58 were heat sensitive for continued expression of the transformed phenotype. tsA1499 is an 81-base-pair deletion at 21 map units which is like tsA58 in that it is also heat sensitive for lytic growth, belongs to the A complementation group, and produces rat cell transformants which contain a thermolabile T antigen. Unlike tsA58, however, tsA1499 generated rat cell transformants efficiently at the temperature at which it was lytically defective, and 10 of 17 clones transformed by tsA1499 were cold rather than heat sensitive for the continued maintenance of the transformed phenotype. The lytic and transforming activities of the A region thus appeared to function independently in mutant tsA1499.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams P. J., Mulder C., Van De Voorde A., Warnaar S. O., van der Eb A. J. Transformation of primary rat kidney cells by fragments of simian virus 40 DNA. J Virol. 1975 Oct;16(4):818–823. doi: 10.1128/jvi.16.4.818-823.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwine J. C., Dhar R., Khoury G. A small RNA induced late in simian virus 40 infection can associate with early viral mRNAs. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1379–1383. doi: 10.1073/pnas.77.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N., Beales N., Shenk T., Berg P., di Mayorca G. New region of the simian virus 40 genome required for efficient viral transformation. Proc Natl Acad Sci U S A. 1978 May;75(5):2473–2477. doi: 10.1073/pnas.75.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: temperature sensitivity of the transformed phenotype and retransofrmation by wild-type virus. J Virol. 1978 Mar;25(3):860–870. doi: 10.1128/jvi.25.3.860-870.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Steinbaugh P. J., Erikson R. L. Characterization of the avian sarcoma virus protein p60src. Virology. 1978 Nov;91(1):130–140. doi: 10.1016/0042-6822(78)90361-6. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Cole C. N., Smith A. E., Paucha E., Tegtmeyer P., Rundell K., Berg P. Organization and expression of early genes of simian virus 40. Proc Natl Acad Sci U S A. 1978 Jan;75(1):117–121. doi: 10.1073/pnas.75.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. A., Khoury G., Martin R. G. Phosphorylation of T-antigen and control T-antigen expression in cells transformed by wild-type and tsA mutants of simian virus 40. J Virol. 1979 Feb;29(2):753–762. doi: 10.1128/jvi.29.2.753-762.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck M. M., Benjamin T. L. Comparisons of two early gene functions essential for transformation in polyoma virus and SV-40. Virology. 1979 Jul 15;96(1):205–228. doi: 10.1016/0042-6822(79)90185-5. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Igel H. J., Price P. J. I. In vitrol transformation of rat embryo cells: correlations with the known tumorigenic activities of chemicals in rodents. In Vitro. 1975 Mar-Apr;11(2):107–116. doi: 10.1007/BF02624083. [DOI] [PubMed] [Google Scholar]
- Gaudray P., Rassoulzadegan M., Cuzin F. Expression of simian virus 40 early genes in transformed rat cells is correlated with maintenance of the transformed phenotype. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4987–4991. doi: 10.1073/pnas.75.10.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y., Davison J., Oren M., Winocour E. Properties of permissive monkey cells transformed by UV-irradiated simian virus 40. J Virol. 1977 May;22(2):256–266. doi: 10.1128/jvi.22.2.256-266.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley S., Bender M. A., Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: effect of transformation technique on the transformed phenotype. J Virol. 1980 Jan;33(1):550–552. doi: 10.1128/jvi.33.1.550-552.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura G., Dulbecco R. A temperature-sensitive mutant of simian virus 40 affecting transforming ability. Virology. 1973 Apr;52(2):529–534. doi: 10.1016/0042-6822(73)90348-6. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A. Initiation and maintenance of cell transformation by simian virus 40: a viral genetic property. Proc Natl Acad Sci U S A. 1975 Feb;72(2):673–677. doi: 10.1073/pnas.72.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lee T. N., Nathans D. A transcriptional map of the SV40 genome in transformed cell lines. Virology. 1975 Jan;63(1):263–272. doi: 10.1016/0042-6822(75)90390-6. [DOI] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Martin R. G. Oncogenicity of simian virus 40 deletion mutants that induce altered 17-kilodalton t-proteins. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4299–4302. doi: 10.1073/pnas.76.9.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Mark D. F., Berg P. A third splice site in SV40 early mRNA. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):55–62. doi: 10.1101/sqb.1980.044.01.008. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A. Roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells: studies with simian virus 40 double mutants. J Virol. 1979 Sep;31(3):596–607. doi: 10.1128/jvi.31.3.596-607.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill F. J., Cohen S., Renzetti L. Temperature dependency for maintenance of transformation in mouse cells transformed by simian virus 40 tsA mutants. J Virol. 1980 Jul;35(1):233–245. doi: 10.1128/jvi.35.1.233-245.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintel D., Bouck N., di Mayorca G., Thimmappaya B., Swerdlow B., Shenk T. SV40 mutant tsA1499 is heat-sensitive for lytic growth but generates cold-sensitive rat-cell transformants. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):305–309. doi: 10.1101/sqb.1980.044.01.035. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cuzin F. Transformation of rat fibroblast cells with early mutants of polyoma (tsa) and simian virus 40 (tsA30): occurrence of either A or N transformants depends on the multiplicity of infection. J Virol. 1980 Feb;33(2):909–911. doi: 10.1128/jvi.33.2.909-911.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Perbal B., Cuzin F. Growth control in simian virus 40-transformed rat cells: temperature-independent expression of the transformed phenotype in tsA transformants derived by agar selection. J Virol. 1978 Oct;28(1):1–5. doi: 10.1128/jvi.28.1.1-5.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rundell K., Collins J. K., Tegtmeyer P., Ozer H. L., Lai C. J., Nathans D. Identification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):636–646. doi: 10.1128/jvi.21.2.636-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K. Product of in vitro translation of the Rous sarcoma virus src gene has protein kinase activity. J Virol. 1979 Apr;30(1):311–318. doi: 10.1128/jvi.30.1.311-318.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K. Temperature-sensitive transformation by Rous sarcoma virus and temperature-sensitive protein kinase activity. J Virol. 1980 Jan;33(1):220–229. doi: 10.1128/jvi.33.1.220-229.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Martin R. G. Growth state of the cell early after infection with simian virus 40 determines whether the maintenance of transformation will be A-gene dependent or independent. J Virol. 1979 Aug;31(2):350–359. doi: 10.1128/jvi.31.2.350-359.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Martin R. G. Simian virus 40 small t antigen is not required for the maintenance of transformation but may act as a promoter (cocarcinogen) during establishment of transformation in resting rat cells. J Virol. 1979 Dec;32(3):979–988. doi: 10.1128/jvi.32.3.979-988.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. Construction of a viable SV40 variant containing two functional origins of DNA replication. Cell. 1978 Apr;13(4):791–798. doi: 10.1016/0092-8674(78)90229-5. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Soule H. R., Lanford R. E., Butel J. S. Antigenic and immunogenic characteristics of nuclear and membrane-associated simian virus 40 tumor antigen. J Virol. 1980 Feb;33(2):887–901. doi: 10.1128/jvi.33.2.887-901.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenen D. G., Martin R. G., Anderson J., Livingston D. M. Biological and biochemical studies of cells transformed by simian virus 40 temperature-sensitive gene A mutants and A mutant revertants. J Virol. 1977 Apr;22(1):210–218. doi: 10.1128/jvi.22.1.210-218.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
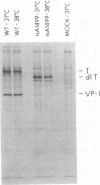
- Yang Y. C., Hearing P., Rundell K. Cellular proteins associated with simian virus 40 early gene products in newly infected cells. J Virol. 1979 Oct;32(1):147–154. doi: 10.1128/jvi.32.1.147-154.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]