Abstract
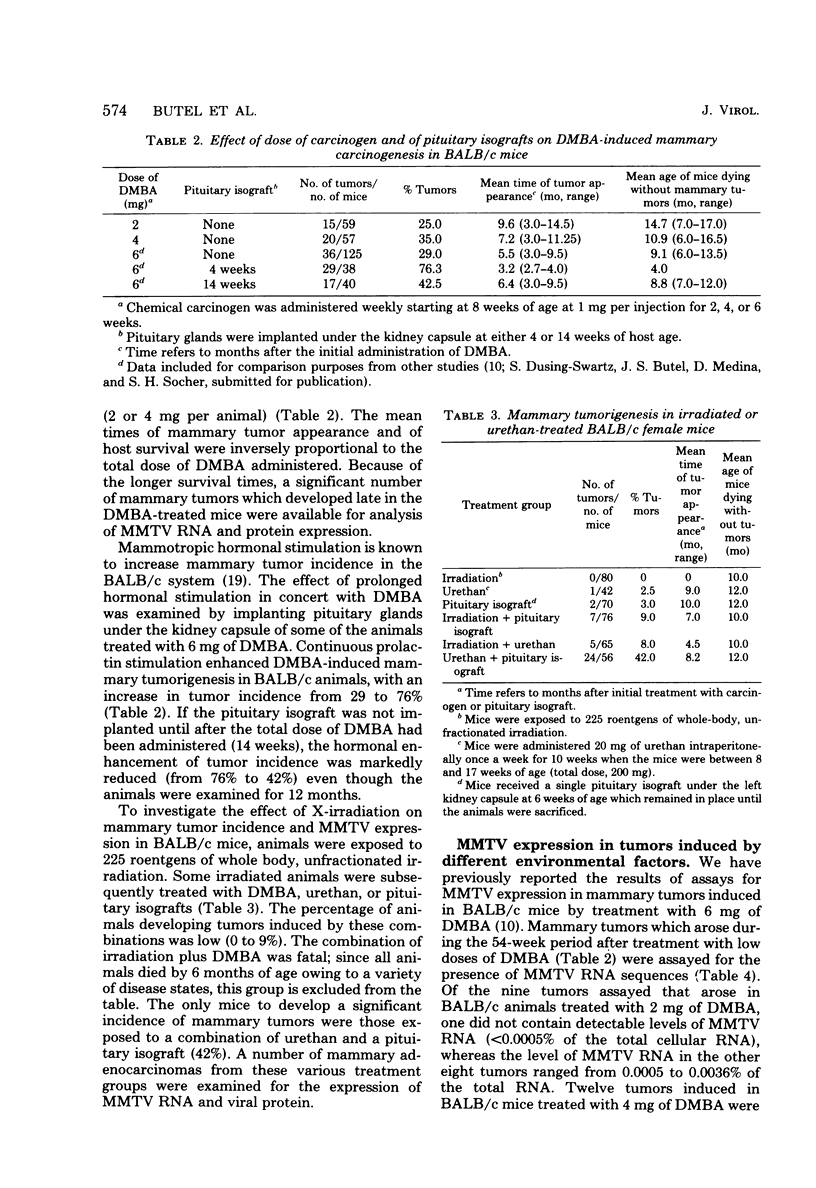
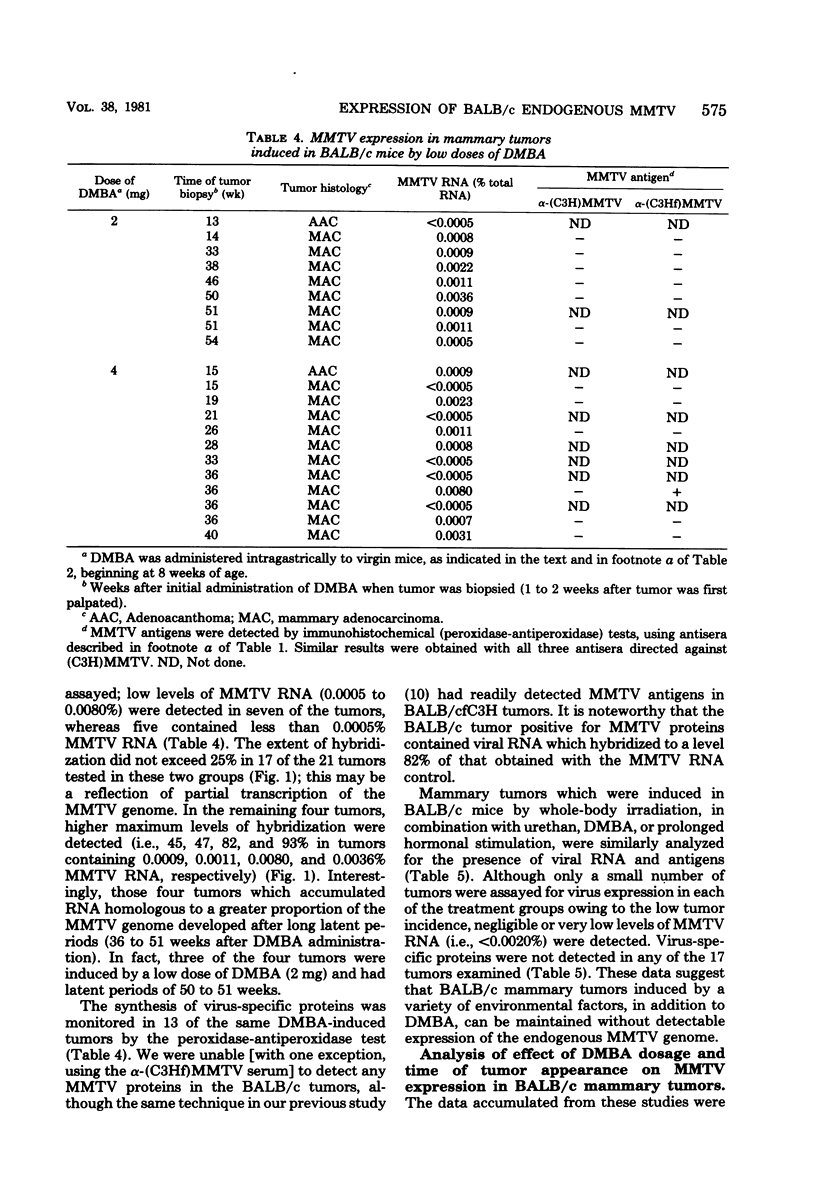
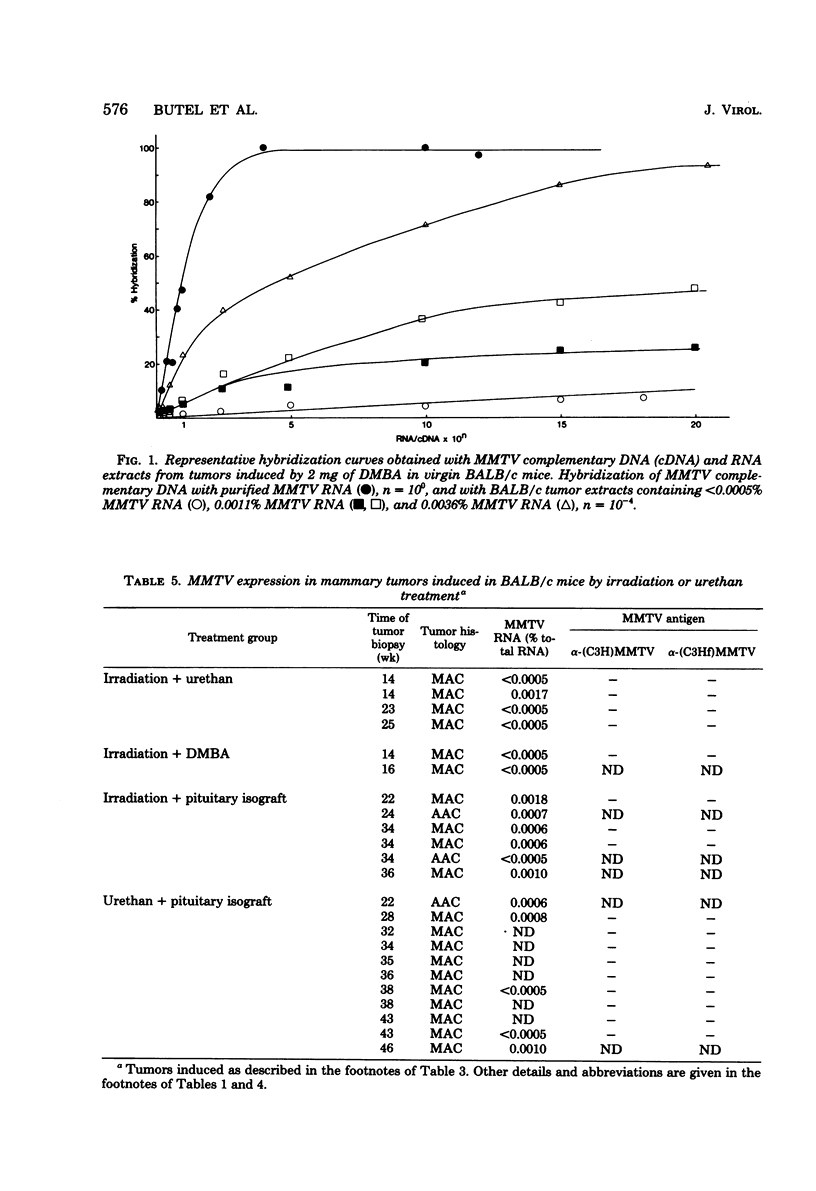
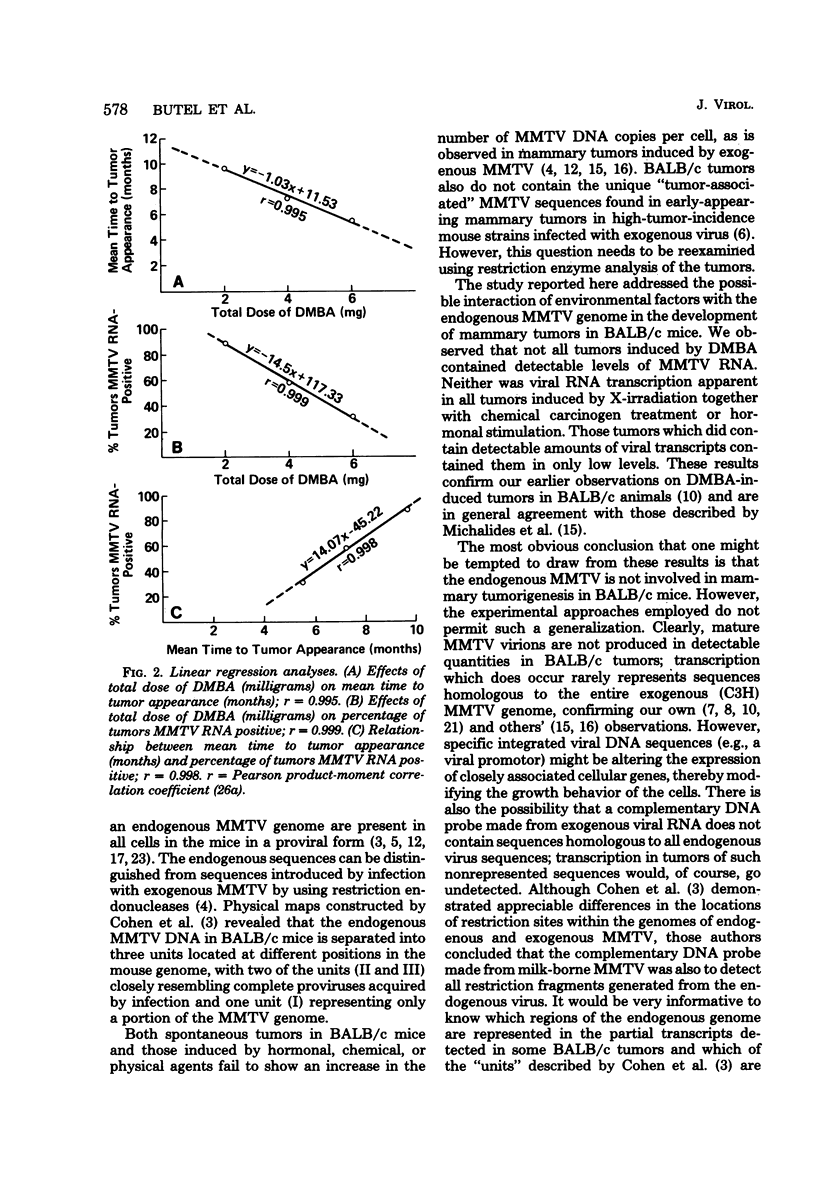
The possible interaction of environmental factors with the endogenous mouse mammary tumor virus (MMTV) genome in the development of mammary tumors in the low-tumor-incidence BALB/c mouse strain was examined. Tumors were induced in virgin female animals by treatment with chemical carcinogen 7,12- dimethylbenz[α]anthracene or urethan, with or without prolonged hormonal stimulation, or by X-irradiation. Concomitant hormonal stimulation resulted in increased tumor incidences compared with those induced by chemical carcinogen treatment alone. The frequency of tumor induction by irradiation alone or in combination with urethan or prolactin stimulation was very low. MMTV expression in the mammary tumors was assayed by nucleic acid hybridization and by immunohistochemical staining. Depending upon the treatment group, 0 to 89% of the tumors contained detectable levels of MMTV RNA (≥0.0005% of the total cellular RNA). Tumors which contained detectable viral transcripts exhibited only low levels of MMTV RNA, which did not appear to represent the accumulation of RNA sequences homologous to the entire MMTV genome; synthesis of MMTV structural proteins was detected in only one tumor. Viral RNA-positive tumors were generally associated with a longer latent period. MMTV RNA expression occurred in tumors classified histologically as adenoacanthomas, as well as in mammary adenocarcinomas, although the cell types in the adenoacanthomas expressing viral RNA were not identified. It does not appear that expression of the endogenous MMTV genome is required for maintenance of all mammary tumors in BALB/c mice, although partial genome expression undetectable by the methods employed cannot be ruled out. Linear regression analyses were performed. The mean time to tumor appearance and the percentage of tumors which were MMTV RNA positive were found to vary linearly as a function of the total dose of 7,12-dimethylbenz[α]anthracene administered. The percentage of tumors which were MMTV RNA positive was also shown to be linearly related to the mean time to tumor appearance. These relationships provide a basis for predictions in the BALB/c system related to these parameters.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentvelzen P., Daams J. H., Hageman P., Calafat J., Timmermans A. Interactions between viral and genetic factors in the origin of mammary tumors in mice. J Natl Cancer Inst. 1972 Apr;48(4):1089–1094. [PubMed] [Google Scholar]
- Bentvelzen P. Host-virus interactions in murine mammary carcinogenesis. Biochim Biophys Acta. 1974 Dec 31;355(3-4):236–259. doi: 10.1016/0304-419x(74)90012-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Majors J. E., Varmus H. E. Organization of mouse mammary tumor virus-specific DNA endogenous to BALB/c mice. J Virol. 1979 Nov;32(2):483–496. doi: 10.1128/jvi.32.2.483-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Proviruses of mouse mammary tumor virus in normal and neoplastic tissues from GR and C3Hf mouse strains. J Virol. 1980 Aug;35(2):298–305. doi: 10.1128/jvi.35.2.298-305.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drohan W., Schlom J. Diversity of mammary tumor viral genes within the genus Mus, the species Mus musculus, and the strain C3H. J Virol. 1979 Jul;31(1):53–62. doi: 10.1128/jvi.31.1.53-62.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J. P., Butel J. S., Socher S. H., Rosen J. M. Detection of mouse mammary tumor virus RNA in BALB/c tumor cell lines of nonviral etiologies. J Virol. 1978 Dec;28(3):743–752. doi: 10.1128/jvi.28.3.743-752.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J. P., Rosen J. M., Butel J. S. Differential expression of poly(A)-adjacent sequences of mammary tumor virus RNA in murine mammary cells. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5797–5801. doi: 10.1073/pnas.75.12.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusing-Swartz S., Medina D., Butel J. S., Socher S. H. Mouse mammary tumor virus genome expression in chemical carcinogen-induced mammary tumors in low- and high-tumor-incidence mouse strains. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5360–5364. doi: 10.1073/pnas.76.10.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell I. H., Van Ness J., Hahn W. E. Assay of DNA-RNA hybrids by S1 nuclease digestion and adsorption to DEAE-cellulose filters. Nucleic Acids Res. 1978 Jun;5(6):2033–2038. doi: 10.1093/nar/5.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath C. M., Marineau E. J., Voyles B. A. Changes in MuMTV DNA and RNA levels in Balb/c mammary epithelial cells during malignant transformation by exogenous MuTV and by hormones. Virology. 1978 Jun 15;87(2):339–353. doi: 10.1016/0042-6822(78)90139-3. [DOI] [PubMed] [Google Scholar]
- Medina D., Butel J. S., Socher S. H., Miller F. L. Mammary tumorigenesis in 7,12-dimethybenzanthracene-treated C57BL x DBA/2f F1 mice. Cancer Res. 1980 Feb;40(2):368–373. [PubMed] [Google Scholar]
- Medina D. Mammary tumorigenesis in chemical carcinogen-treated mice. I. Incidence in BALB-c and C57BL mice. J Natl Cancer Inst. 1974 Jul;53(1):213–221. doi: 10.1093/jnci/53.1.213. [DOI] [PubMed] [Google Scholar]
- Michalides R., van Deemter L., Nusse R., Hageman P. Induction of mouse mammary tumor virus RNA in mammary tumors of BALB/c mice treated with urethane, X-irradiation, and hormones. J Virol. 1979 Jul;31(1):63–72. doi: 10.1128/jvi.31.1.63-72.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., van Deemter L., Nusse R., Röpcke G., Boot L. Involvement of mouse mammary tumor virus in spontaneous and hormone-induced mammary tumors in low-mammary-tumor mouse strains. J Virol. 1978 Sep;27(3):551–559. doi: 10.1128/jvi.27.3.551-559.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris V. L., Medeiros E., Ringold G. M., Bishop J. M., Varmus H. E. Comparison of mouse mammary tumor virus-specific DNA in inbred, wild and Asian mice, and in tumors and normal organs from inbred mice. J Mol Biol. 1977 Jul;114(1):73–91. doi: 10.1016/0022-2836(77)90284-4. [DOI] [PubMed] [Google Scholar]
- Ono T., Cutler R. G. Age-dependent relaxation of gene repression: increase of endogenous murine leukemia virus-related and globin-related RNA in brain and liver of mice. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4431–4435. doi: 10.1073/pnas.75.9.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley R. J., Medina D., Socher S. H. Murine mammary tumor virus expression during mammary tumorigenesis in BALB/c mice. J Virol. 1979 Feb;29(2):483–493. doi: 10.1128/jvi.29.2.483-493.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley R. J., Rosen J. M., Socher S. H. Mammary tumour virus and casein gene transcription during mouse mammary development. Nature. 1978 Oct 5;275(5679):455–457. doi: 10.1038/275455a0. [DOI] [PubMed] [Google Scholar]
- Schlom J., Colcher D., Drohan W., Kettmann R., Michalides R., Vlahakis G., Young J. Differences in mouse mammary tumor viruses. Relationship to early and late occurring mammary tumors. Cancer. 1977 Jun;39(6 Suppl):2727–2733. doi: 10.1002/1097-0142(197706)39:6<2727::aid-cncr2820390660>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Schlom J., Michalides R., Kufe D., Hehlmann R., Spiegelman S., Bentvelzen P., Hageman P. A comparative study of the biologic and molecular basis of murine mammary carcinoma: a model for human breast cancer. J Natl Cancer Inst. 1973 Aug;51(2):541–551. [PubMed] [Google Scholar]
- Smith G. H., Arthur L. A., Medina D. Evidence of separate pathways for viral and chemical carcinogenesis in C3H/StWi mouse mammary glands. Int J Cancer. 1980 Sep 15;26(3):373–379. doi: 10.1002/ijc.2910260318. [DOI] [PubMed] [Google Scholar]
- Smith G. H., Pauley R. J., Socher S. H., Medina D. Chemical carcinogenesis in C3H/StWi mice, a worthwhile experimental model for breast cancer. Cancer Res. 1978 Dec;38(12):4504–4509. [PubMed] [Google Scholar]
- Teramoto Y. A., Medina D., McGrath C., Schlom J. Noncoordinate expression of murine mammary tumor virus gene products. Virology. 1980 Dec;107(2):345–353. doi: 10.1016/0042-6822(80)90302-5. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Medeiros E., Bishop J. M., Nowinski R. C., Sarkar N. H. Transcription of mouse mammary tumor virus genes in tissues from high and low tumor incidence mouse strains. J Mol Biol. 1973 Oct 5;79(4):663–679. doi: 10.1016/0022-2836(73)90070-3. [DOI] [PubMed] [Google Scholar]
- Young B. D., Harrison P. R., Gilmour R. S., Birnie G. D., Hell A., Humphries S., Paul J. Kinetic studies of gene frequency. II. Complexity of globin complementary DNA and its hybridization characteristics. J Mol Biol. 1974 Apr 25;84(4):555–568. doi: 10.1016/0022-2836(74)90116-8. [DOI] [PubMed] [Google Scholar]


