Abstract
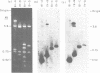
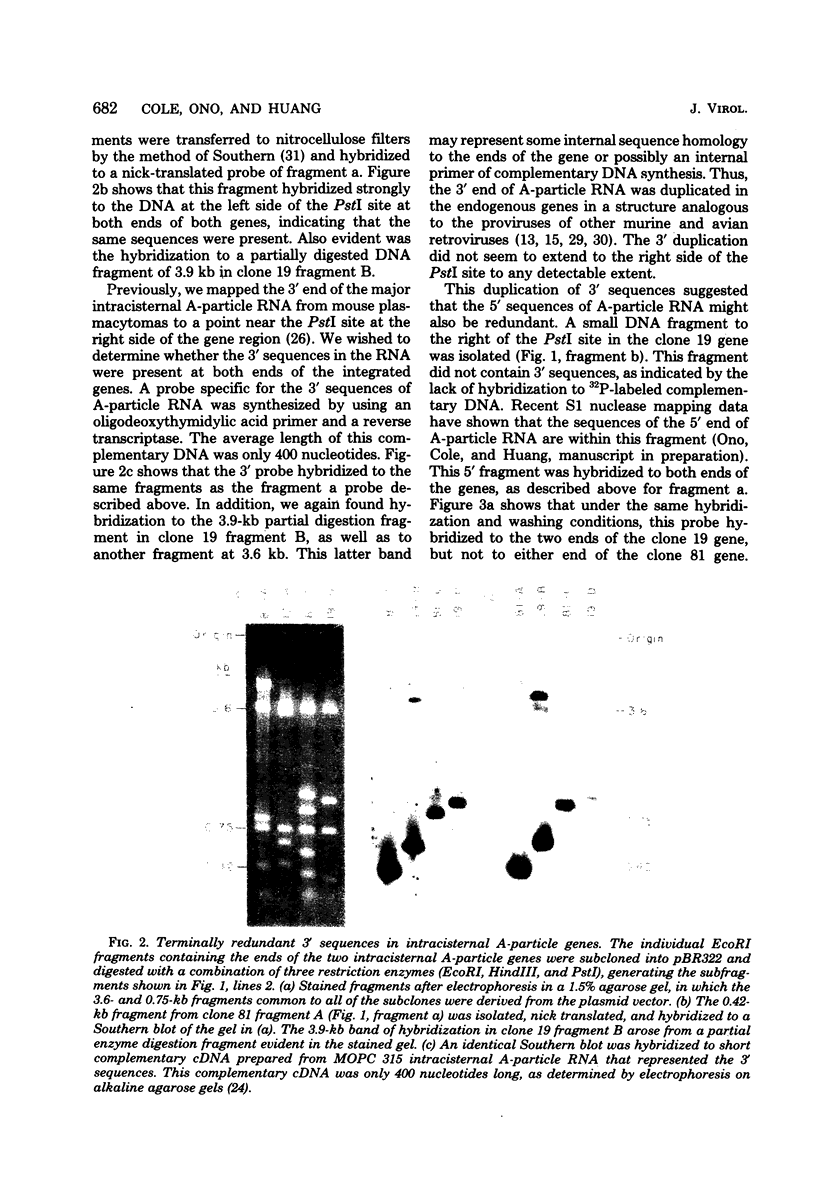
The sequences coding for intracisternal A-particle RNA form a family of related but not identical genetic elements which are present in 650 to 1,000 copies within the mouse genome. We showed that different intracisternal A-particle genes had a terminally redundant sequence of about 400 base pairs, one-half of which arose from the 3' end of the intracisternal A-particle RNA. A second portion of the redundant region did not contain 3-related sequences and was probably derived from the 5' end of intracisternal A-particle RNA. Thus, there were endogenous intracisternal A-particle genes in the cellular DNA-3'-5'--3'-5'-cellular DNA configuration identified for type B and C retroviruses. This indicated that the initial integration of intracisternal A-particle genes into the Mus musculus genome occurred by the same mechanism as the integration of other retroviruses. Two types of heterogeneity were identified among the 5' sequences of the two genes.
Full text
PDF


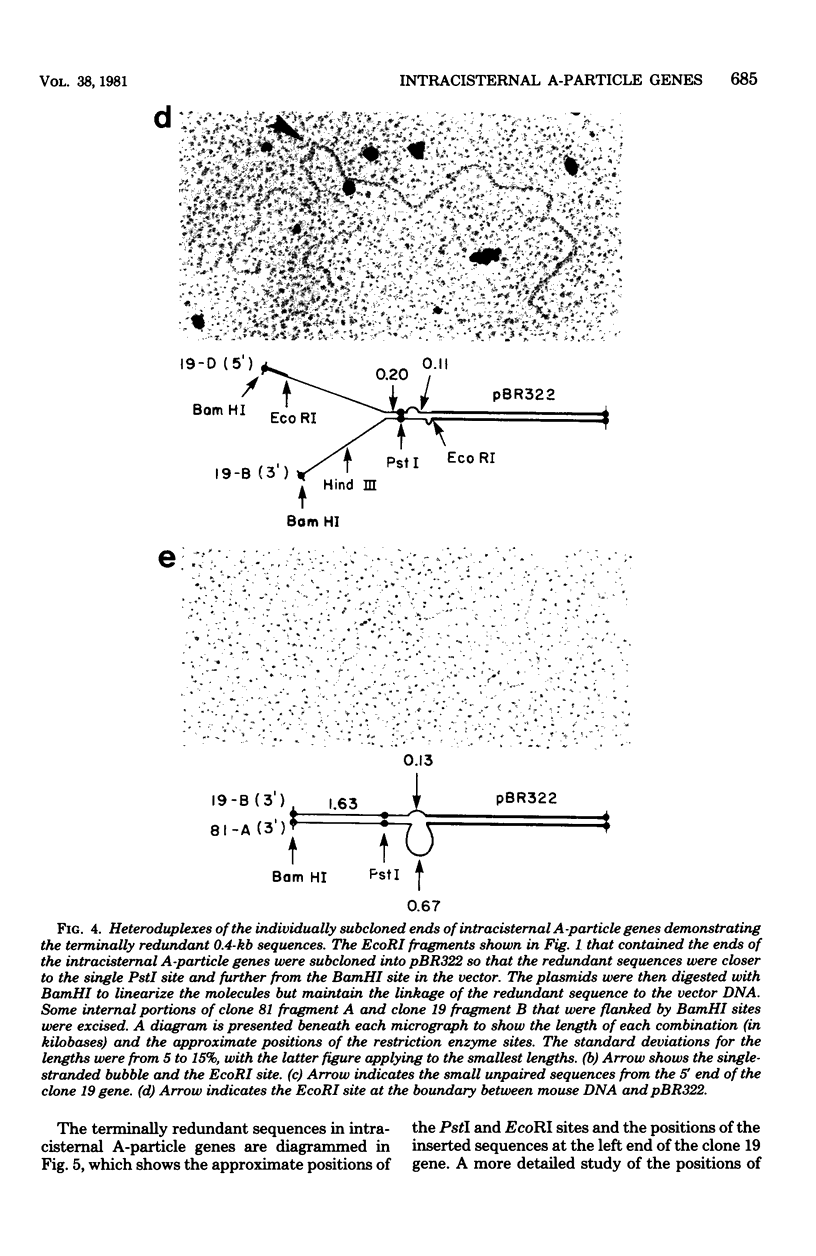


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biczysko W., Pienkowski M., Solter D., Koprowski H. Virus particles in early mouse embryos. J Natl Cancer Inst. 1973 Sep;51(3):1041–1050. doi: 10.1093/jnci/51.3.1041. [DOI] [PubMed] [Google Scholar]
- Calarco P. G., Szollosi D. Intracisternal A particles in ova and preimplantation stages of the mouse. Nat New Biol. 1973 May 16;243(124):91–93. [PubMed] [Google Scholar]
- Cameron J. R., Loh E. Y., Davis R. W. Evidence for transposition of dispersed repetitive DNA families in yeast. Cell. 1979 Apr;16(4):739–751. doi: 10.1016/0092-8674(79)90090-4. [DOI] [PubMed] [Google Scholar]
- Chase D. G., Pikó L. Expression of A- and C-type particles in early mouse embryos. J Natl Cancer Inst. 1973 Dec;51(6):1971–1975. doi: 10.1093/jnci/51.6.1971. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- DALTON A. J., POTTER M., MERWIN R. M. Some ultrastructural characteristics of a series of primary and transplanted plasma-cell tumors of the mouse. J Natl Cancer Inst. 1961 May;26:1221–1267. [PubMed] [Google Scholar]
- DE HARVEN E., FRIEND C. Electron microscope study of a cell-free induced leukemia of the mouse: a preliminary report. J Biophys Biochem Cytol. 1958 Mar 25;4(2):151–156. doi: 10.1083/jcb.4.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- FRIEDLAENDER M., MOORE D. H. Occurrence of bodies within endoplasmic reticulum of Ehrlich ascites tumor cells. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):828–831. doi: 10.3181/00379727-92-22627. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J., Rubin G. M., Young M. W., Hogness D. S. Repeated gene families in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1053–1063. doi: 10.1101/sqb.1978.042.01.106. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Goff S., Shields A., Yoshimura F., Mitra S., Baltimore D. In vitro synthesis of a 9 kbp terminally redundant DNA carrying the infectivity of Moloney murine leukemia virus. Cell. 1979 Apr;16(4):863–874. doi: 10.1016/0092-8674(79)90101-6. [DOI] [PubMed] [Google Scholar]
- Hall W. T., Hartley J. W., Sanford K. K. Characteristics of and relationship between C particles and intracisternal A particles in cloned cell strains. J Virol. 1968 Mar;2(3):238–247. doi: 10.1128/jvi.2.3.238-247.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Kakefuda T., Roberts E., Suntzeff V. Electron microscopic study of methylcholanthrene-induced epidermal carcinogenesis in mice: mitochondrial dense bodies and intracisternal A-particles. Cancer Res. 1970 Apr;30(4):1011–1019. [PubMed] [Google Scholar]
- Keshet E., Shaul Y., Kaminchik J., Aviv H. Heterogeneity of "virus-like" genes encoding retrovirus-associated 30S RNA and their organization within the mouse genome. Cell. 1980 Jun;20(2):431–439. doi: 10.1016/0092-8674(80)90629-7. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Translocatable elements in procaryotes. Cell. 1977 May;11(1):11–23. doi: 10.1016/0092-8674(77)90313-0. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K., Scolnick E. M. Nucleotide sequence relationship between intracisternal type A particles of Mus musculus and an endogenous retrovirus (M432) of Mus cervicolor. J Virol. 1978 Oct;28(1):66–74. doi: 10.1128/jvi.28.1.66-74.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Wivel N. A., Lueders K. K. The extraction of intracisternal A-particles from a mouse plasma-cell tumor. Cancer Res. 1968 Oct;28(10):2137–2148. [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Genetic individuality of intracisternal A-particles of Mus musculus. J Virol. 1979 Apr;30(1):225–231. doi: 10.1128/jvi.30.1.225-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Intracisternal A-particle genes: identification in the genome of Mus musculus and comparison of multiple isolates from a mouse gene library. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3571–3575. doi: 10.1073/pnas.77.6.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Sequences associated with intracisternal A particles are reiterated in the mouse genome. Cell. 1977 Dec;12(4):963–972. doi: 10.1016/0092-8674(77)90161-1. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Ono M., Cole M. D., White A. T., Huang R. C. Sequence organization of cloned intracisternal A particle genes. Cell. 1980 Sep;21(2):465–473. doi: 10.1016/0092-8674(80)90483-3. [DOI] [PubMed] [Google Scholar]
- Potter S. S., Brorein W. J., Jr, Dunsmuir P., Rubin G. M. Transposition of elements of the 412, copia and 297 dispersed repeated gene families in Drosophila. Cell. 1979 Jun;17(2):415–427. doi: 10.1016/0092-8674(79)90168-5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sabran J. L., Hsu T. W., Yeater C., Kaji A., Mason W. S., Taylor J. M. Analysis of integrated avian RNA tumor virus DNA in transformed chicken, duck and quail fibroblasts. J Virol. 1979 Jan;29(1):170–178. doi: 10.1128/jvi.29.1.170-178.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Reitz M. S., Jr, Trainor C. D., Gallo R. C. Murine intracisternal type A particles: a biochemical characterization. J Virol. 1975 Oct;16(4):887–896. doi: 10.1128/jvi.16.4.887-896.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]