Abstract
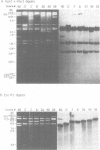
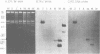
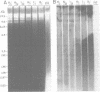
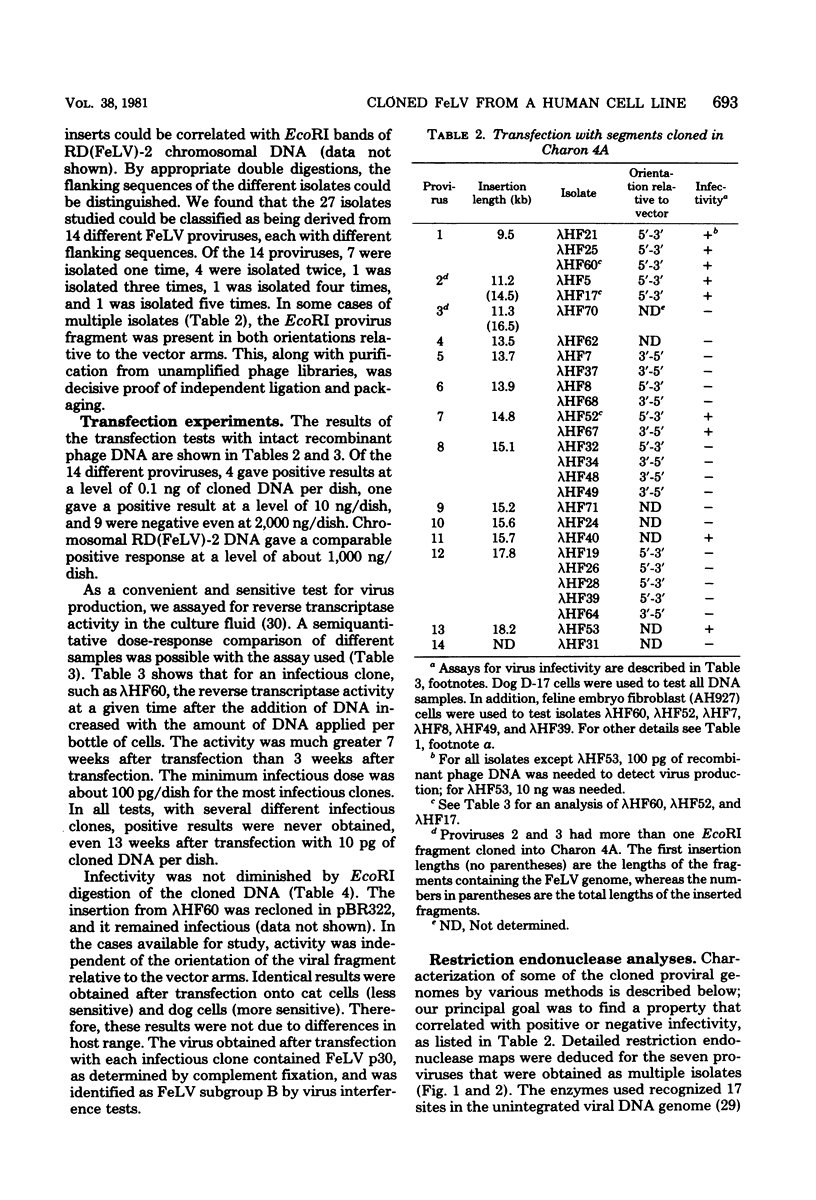
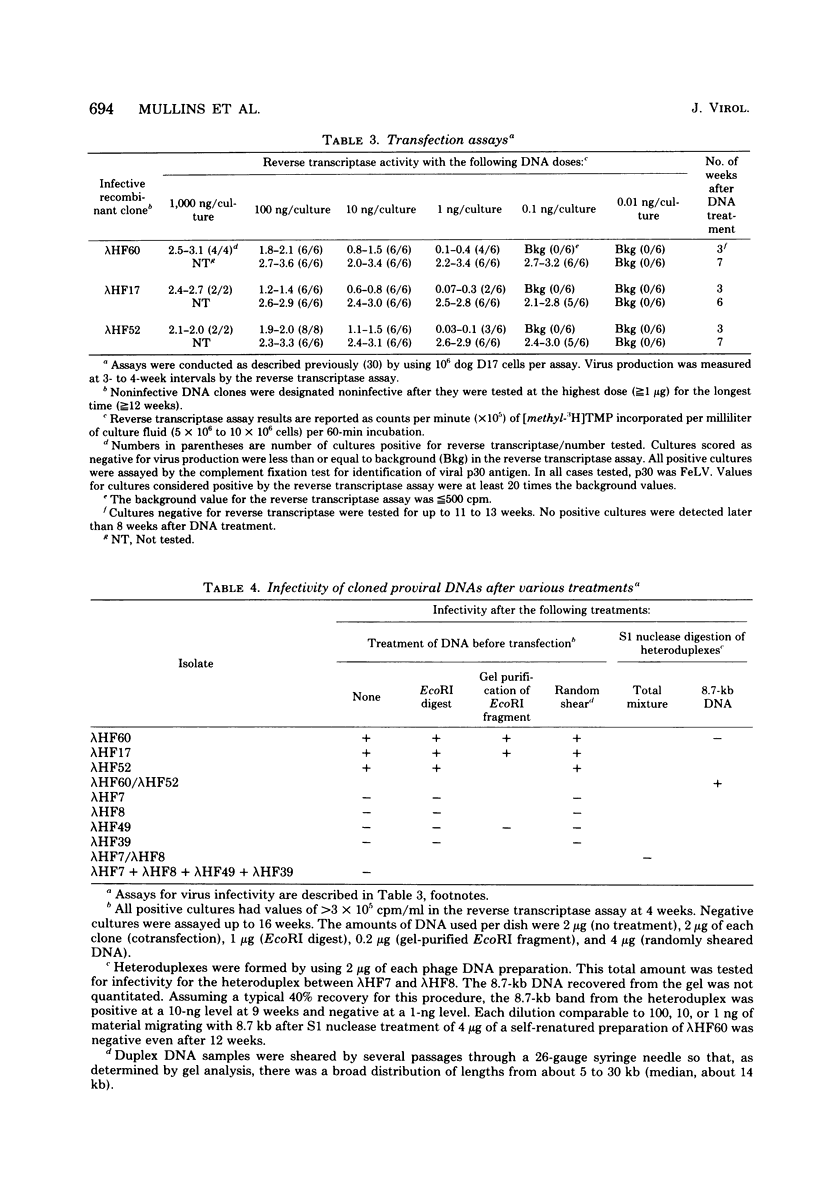
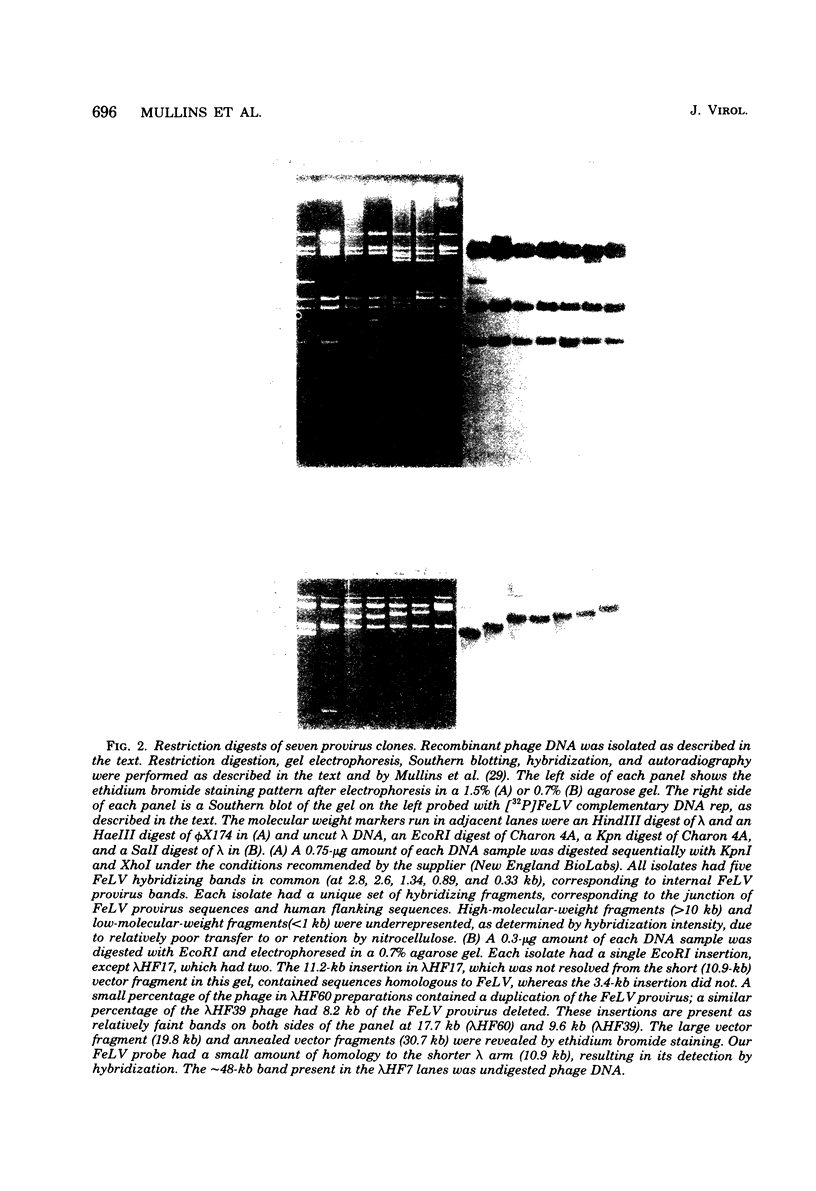
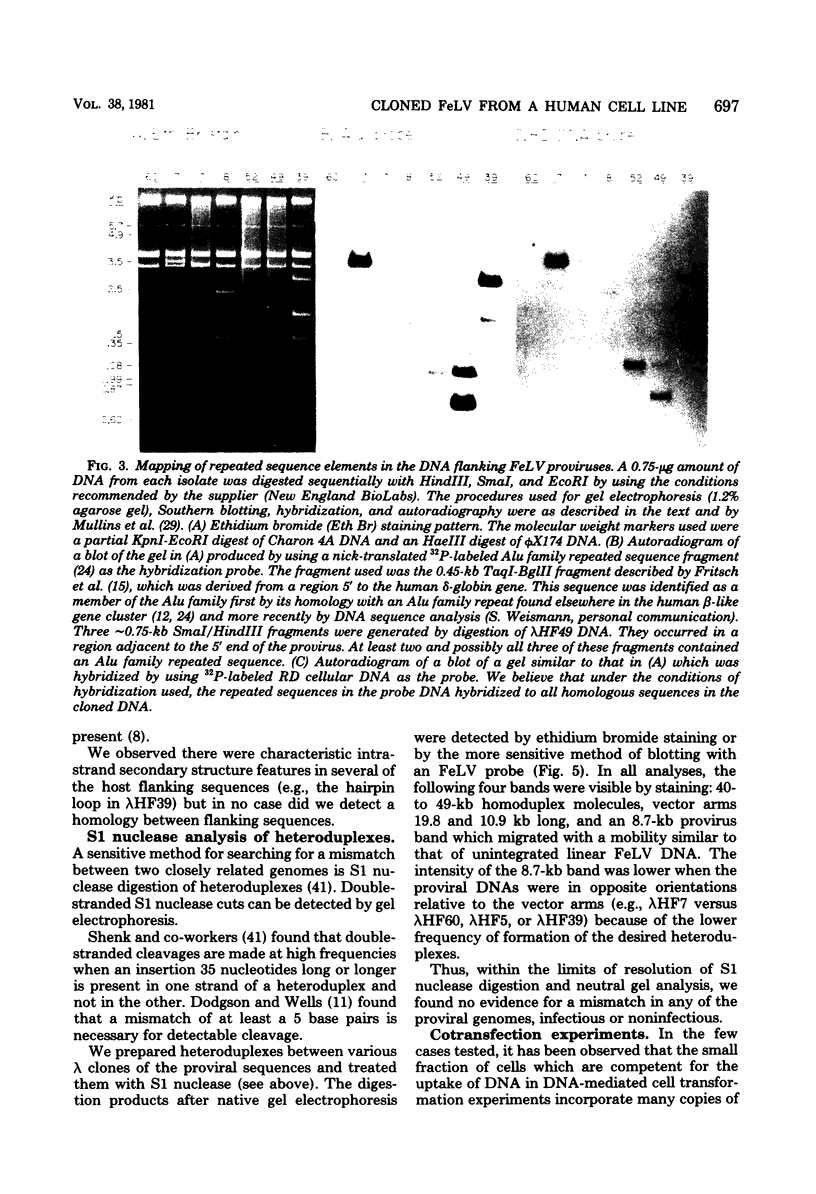
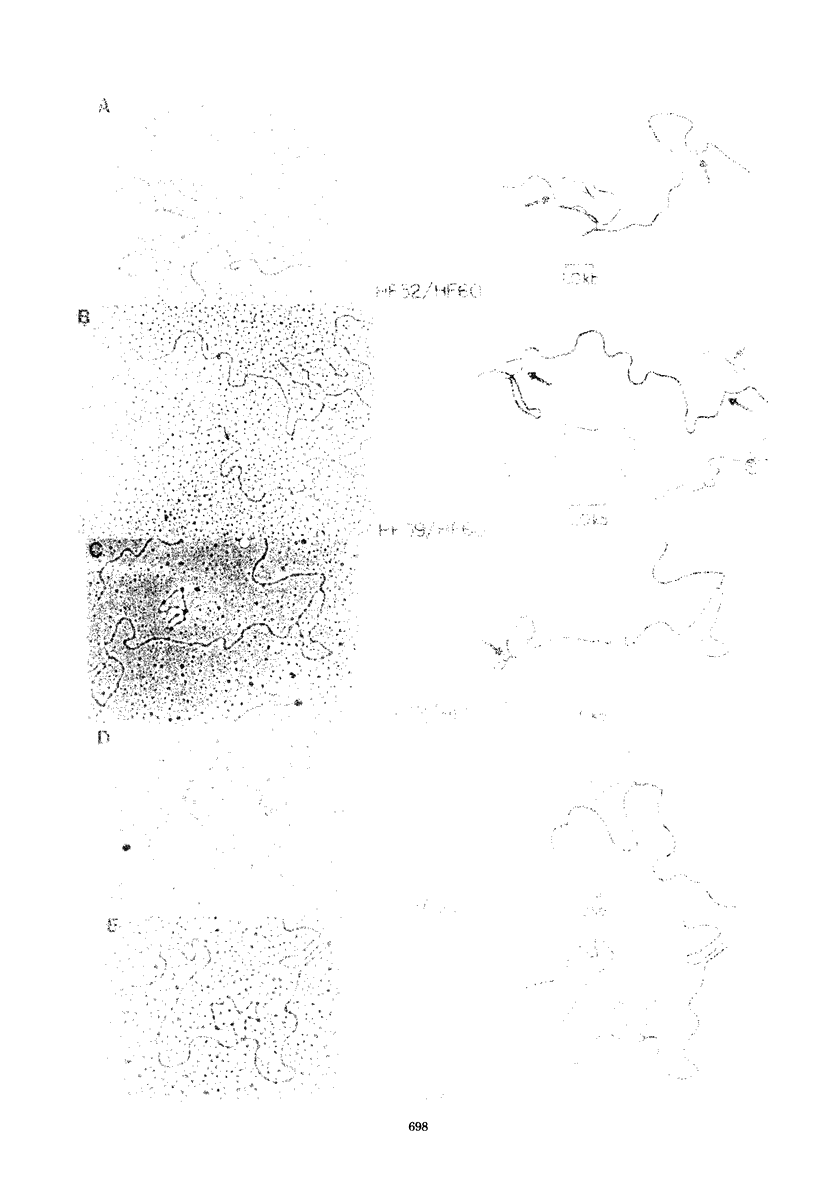
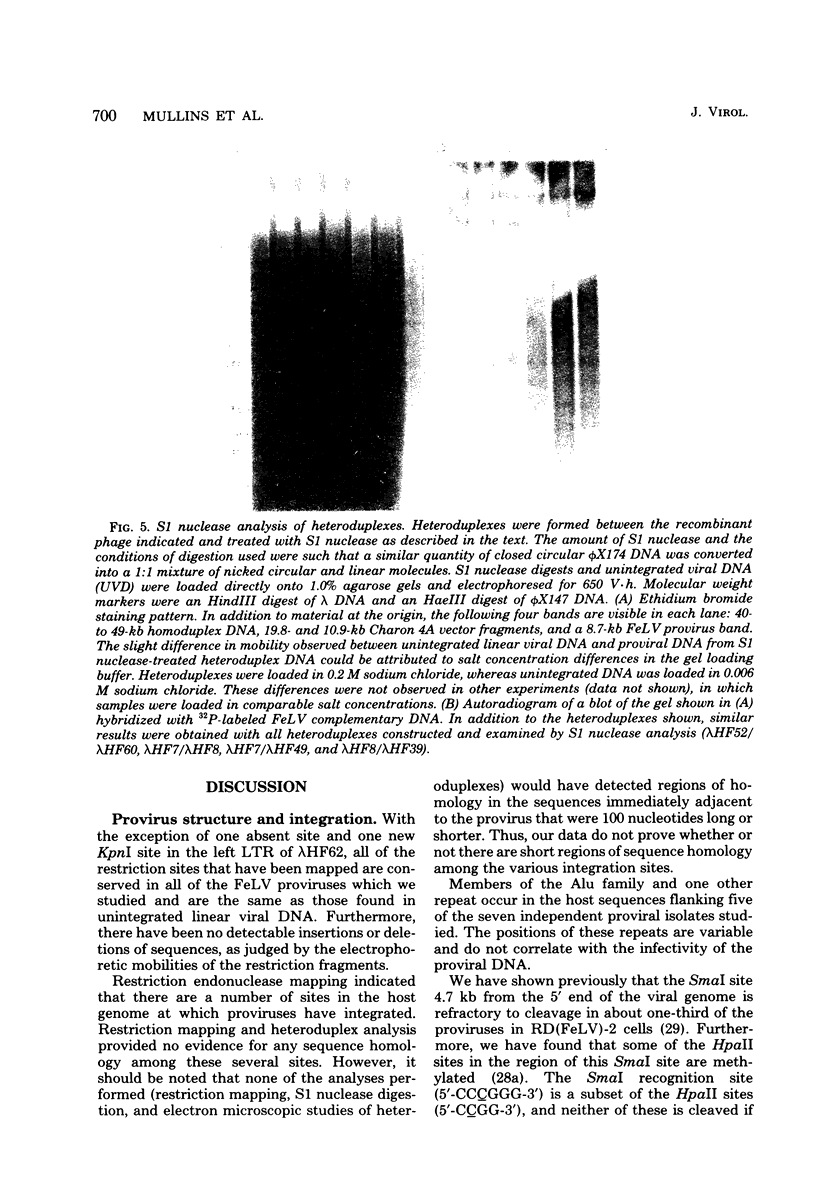
We examined 14 different feline leukemia virus proviruses from the productively infected human cell line RD(FeLV)-2 after cloning in the modified lambda vector Charon 4A. Each isolate was characterized by restriction digestion and Southern blot analysis. The DNA of each isolate was tested for competence to express virus after uptake by sensitive animal cells (transfection). All but one isolate contained an apparently complete provirus, but only four were infectious. Seven isolates (four noninfectious, three infectious) were studied by heteroduplexing followed by electron microscopy or by S1 nuclease treatment and gel electrophoresis. No regions of nonhomology between proviruses were detected by either criterion, and in no case did we observe homology between flanking sequences. Random shearing or removal of flanking sequences by S1 nuclease had no effect on the status of infectivity of the clones. Thus, we were unable to find molecular differences between infectious and noninfectious proviruses. Our data are consistent with either of the following hypotheses: (i) that there is a short host sequence which is essential as a promoter for virus expression; or (ii) that lack of infectivity is due to small mutations within the proviral genome.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Chen C. W., Thomas C. A., Jr Recovery of DNA segments from agarose gels. Anal Biochem. 1980 Jan 15;101(2):339–341. doi: 10.1016/0003-2697(80)90197-9. [DOI] [PubMed] [Google Scholar]
- Collins J., Hohn B. Cosmids: a type of plasmid gene-cloning vector that is packageable in vitro in bacteriophage lambda heads. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4242–4246. doi: 10.1073/pnas.75.9.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Silverman L. Linkage of the endogenous avian leukosis virus genome of virus-producing chicken cells to inhibitory cellular DNA sequences. Cell. 1978 Oct;15(2):573–577. doi: 10.1016/0092-8674(78)90025-9. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Infectious rous sarcoma virus and reticuloendotheliosis virus DNAs. J Virol. 1974 Nov;14(5):1132–1141. doi: 10.1128/jvi.14.5.1132-1141.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Lack of infectivity of the endogenous avian leukosis virus-related genes in the DNA of uninfected chicken cells. J Virol. 1976 Feb;17(2):422–430. doi: 10.1128/jvi.17.2.422-430.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Schmid C. W. An electron microscope study of the DNA sequence organization of the human genome. J Mol Biol. 1976 Sep 25;106(3):773–790. doi: 10.1016/0022-2836(76)90264-3. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Wells R. D. Action of single-strand specific nucleases on model DNA heteroduplexes of defined size and sequence. Biochemistry. 1977 May 31;16(11):2374–2379. doi: 10.1021/bi00630a010. [DOI] [PubMed] [Google Scholar]
- Duncan C., Biro P. A., Choudary P. V., Elder J. T., Wang R. R., Forget B. G., de Riel J. K., Weissman S. M. RNA polymerase III transcriptional units are interspersed among human non-alpha-globin genes. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5095–5099. doi: 10.1073/pnas.76.10.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex M., Cotter S. M., Hardy W. D., Jr, Hess P., Jarrett W., Jarrett O., Mackey L., Laird H., Perryman L., Olsen R. G. Feline oncornavirus-associated cell membrane antigen. IV. Antibody titers in cats with naturally occurring leukemia, lymphoma, and other diseases. J Natl Cancer Inst. 1975 Aug;55(2):463–467. [PubMed] [Google Scholar]
- Filbert J. E., McAllister R. M., Nicolson M. O., Gilden R. V., Lennette E. H. RD-114 virus infectivity assay by measurements of DNA polymerase activity and virus group specific antigen. Proc Soc Exp Biol Med. 1974 Feb;145(2):366–370. doi: 10.3181/00379727-145-37812. [DOI] [PubMed] [Google Scholar]
- Gautier F., Bünemann H., Grotjahn L. Analysis of calf-thymus satellite DNA: evidence for specific methylation of cytosine in C-G sequences. Eur J Biochem. 1977 Oct 17;80(1):175–183. doi: 10.1111/j.1432-1033.1977.tb11869.x. [DOI] [PubMed] [Google Scholar]
- Hardy W. D., Jr, McClelland A. J., Zuckerman E. E., Snyder H. W., Jr, MacEwen E. G., Francis D., Essex M. Development of virus non-producer lymphosarcomas in pet cats exposed to FeLv. Nature. 1980 Nov 6;288(5786):90–92. doi: 10.1038/288090a0. [DOI] [PubMed] [Google Scholar]
- Hill M., Hillova J. Virus recovery in chicken cells tested with Rous sarcoma cell DNA. Nat New Biol. 1972 May 10;237(71):35–39. doi: 10.1038/newbio237035a0. [DOI] [PubMed] [Google Scholar]
- Hill M., Hillová J. Production virale dans les fibroblastes de poule traités par l'acide désoxyribonucléique de cellulex XC de rat transformées par le virus de Rous. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jun 14;272(24):3094–3097. [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck C. M., Rinehart F. P., Schmid C. W. A ubiquitous family of repeated DNA sequences in the human genome. J Mol Biol. 1979 Aug 15;132(3):289–306. doi: 10.1016/0022-2836(79)90261-4. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Hardy W. D., Jr, Golder M. C., Hay D. The frequency of occurrence of feline leukaemia virus subgroups in cats. Int J Cancer. 1978 Mar 15;21(3):334–337. doi: 10.1002/ijc.2910210314. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Laird H. M., Hay D., Crighton G. W. Replication of cat leukemia virus in cell cultures. Nature. 1968 Aug 3;219(5153):521–522. doi: 10.1038/219521a0. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis J. T., Prestidge L., Hogness D. S. A novel arrangement of tandemly repeated genes at a major heat shock site in D. melanogaster. Cell. 1978 Aug;14(4):901–919. doi: 10.1016/0092-8674(78)90345-8. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Mullins J. I., Casey J. W., Nicolson M. O., Davidson N. Sequence organization of feline leukemia virus DNA in infected cells. Nucleic Acids Res. 1980 Aug 11;8(15):3287–3305. doi: 10.1093/nar/8.15.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson M. O., Hariri F., Krempin H. M., McAllister R. M., Gilden R. V. Infectious proviral DNA in human cells infected with transformation-defective type C viruses. Virology. 1976 Apr;70(2):301–312. doi: 10.1016/0042-6822(76)90273-7. [DOI] [PubMed] [Google Scholar]
- O'Rear J. J., Mizutani S., Hoffman G., Fiandt M., Temin H. M. Infectious and noninfectious recombinant clones of the provirus of SNV differ in cellular DNA and are apparently the same in viral DNA. Cell. 1980 Jun;20(2):423–430. doi: 10.1016/0092-8674(80)90628-5. [DOI] [PubMed] [Google Scholar]
- O'Rear J. J., Mizutani S., Hoffman G., Fiandt M., Temin H. M. Infectious and noninfectious recombinant clones of the provirus of SNV differ in cellular DNA and are apparently the same in viral DNA. Cell. 1980 Jun;20(2):423–430. doi: 10.1016/0092-8674(80)90628-5. [DOI] [PubMed] [Google Scholar]
- Okabe H., DuBuy J., Gilden R. V., Gardner M. B. A portion of the feline leukaemia virus genome is not endogenous in cat cells. Int J Cancer. 1978 Jul 15;22(1):70–78. doi: 10.1002/ijc.2910220114. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T., Jain D., Hill P. R., Huebner R. J. Differential host range of viruses of feline leukemia-sarcoma complex. Virology. 1975 Apr;64(2):438–446. doi: 10.1016/0042-6822(75)90121-x. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology. 1973 Jul;54(1):160–169. doi: 10.1016/0042-6822(73)90125-6. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Tseng J., Lee Y. K., Gilden R. V. Virus similar to RD114 virus in cat cells. Nat New Biol. 1973 Jul 11;244(132):56–59. doi: 10.1038/newbio244056a0. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Deininger P. L. Sequence organization of the human genome. Cell. 1975 Nov;6(3):345–358. doi: 10.1016/0092-8674(75)90184-1. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Rhodes C., Rigby P. W., Berg P. Biochemical method for mapping mutational alterations in DNA with S1 nuclease: the location of deletions and temperature-sensitive mutations in simian virus 40. Proc Natl Acad Sci U S A. 1975 Mar;72(3):989–993. doi: 10.1073/pnas.72.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. A biochemical method for increasing the size of deletion mutations in simian virus 40 DNA. J Mol Biol. 1977 Jul 5;113(3):503–515. doi: 10.1016/0022-2836(77)90235-2. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Oskarsson M., Maizel J., Vande Woude G. Molecular cloning of Snyder-Theilen feline leukemia and sarcoma viruses: comparative studies of feline sarcoma virus with its natural helper virus and with Moloney murine sarcoma virus. J Virol. 1980 Apr;34(1):200–212. doi: 10.1128/jvi.34.1.200-212.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Khan A. S., Sliski A. H., Essex M. Feline oncornavirus-associated cell membrane antigen: evidence for an immunologically crossreactive feline sarcoma virus-coded protein. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5608–5612. doi: 10.1073/pnas.74.12.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Leder P. Purification and cloning of a mouse ribosomal gene fragment in coliphage lambda. Gene. 1977;2(3-4):173–191. doi: 10.1016/0378-1119(77)90016-6. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. MspI, an isoschizomer of hpaII which cleaves both unmethylated and methylated hpaII sites. Nucleic Acids Res. 1978 Sep;5(9):3231–3236. doi: 10.1093/nar/5.9.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. Integrated genomes of animal viruses. Annu Rev Biochem. 1980;49:197–226. doi: 10.1146/annurev.bi.49.070180.001213. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]