Abstract
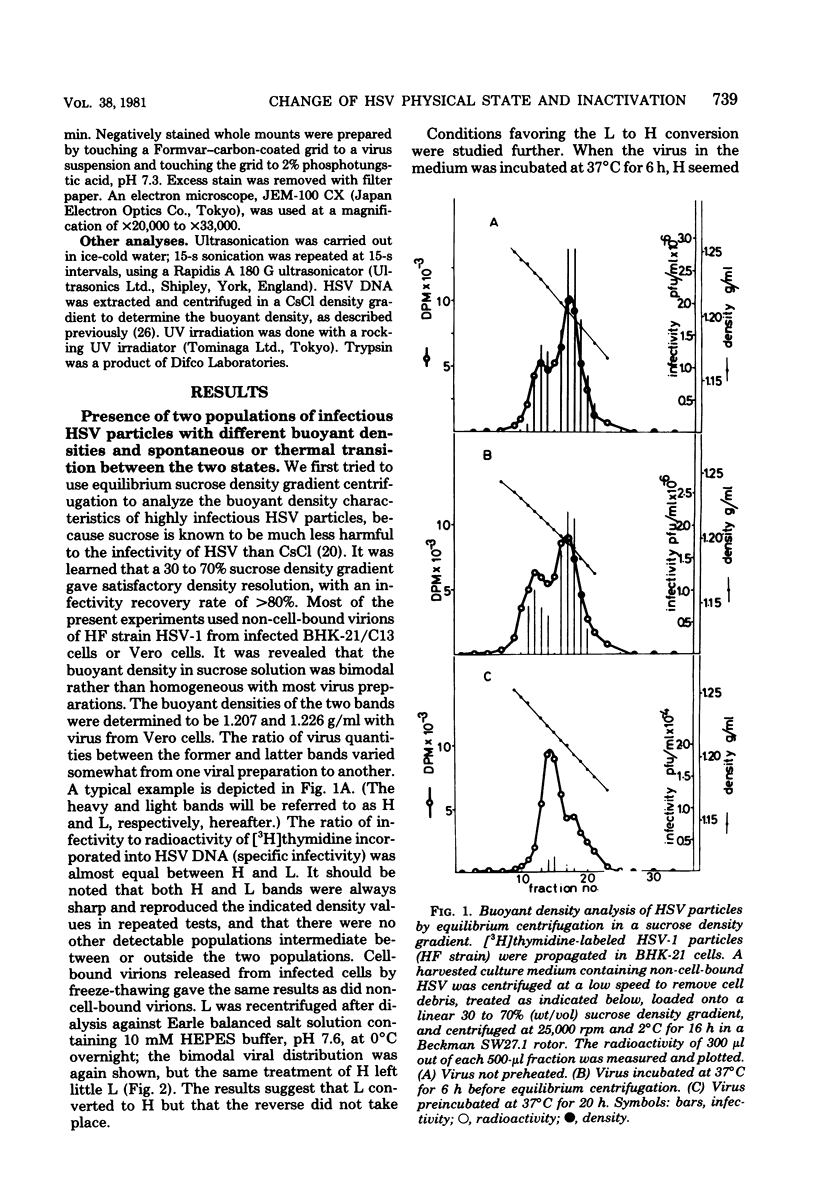
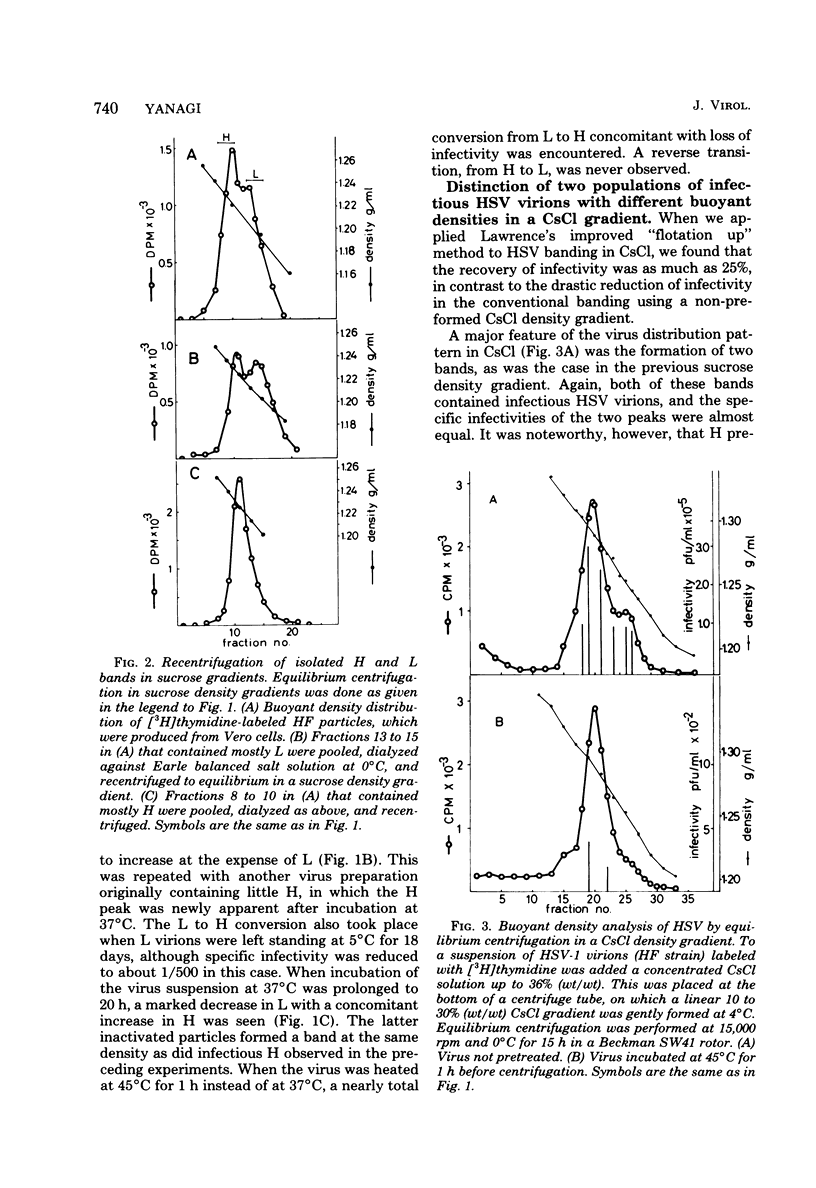
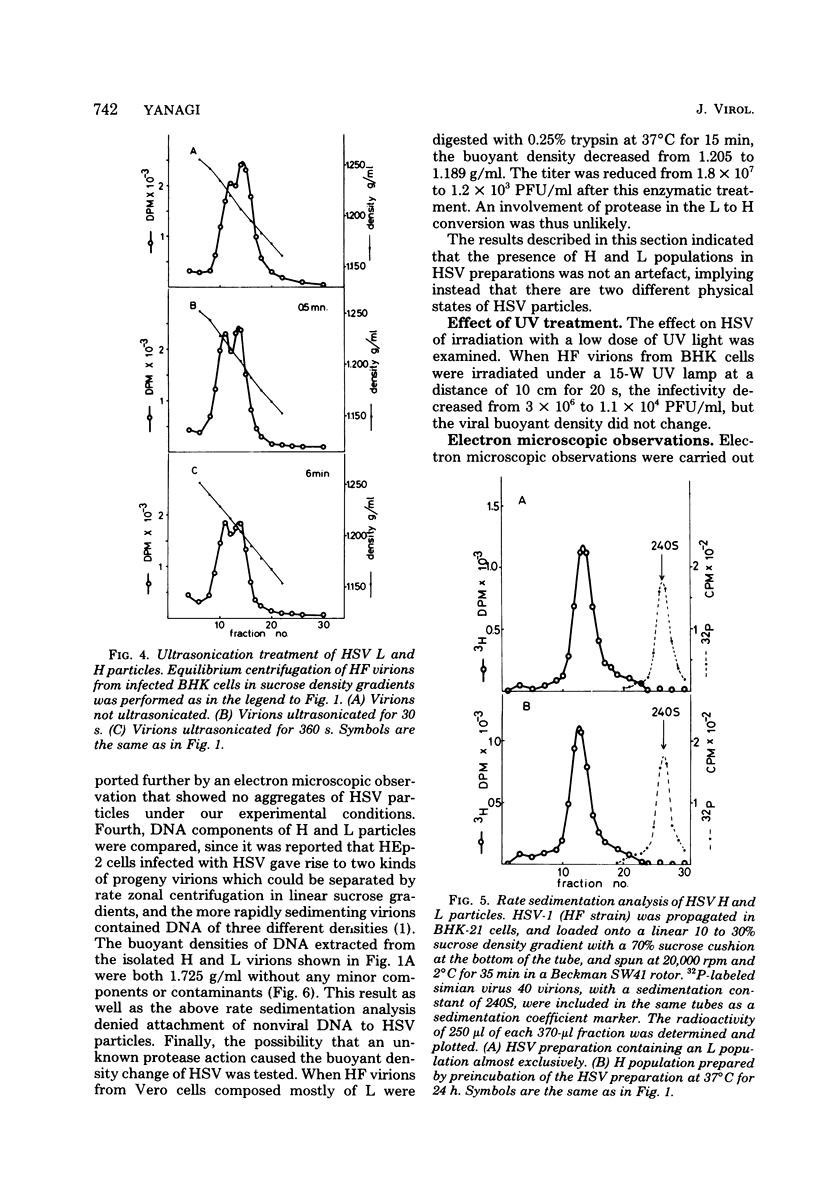
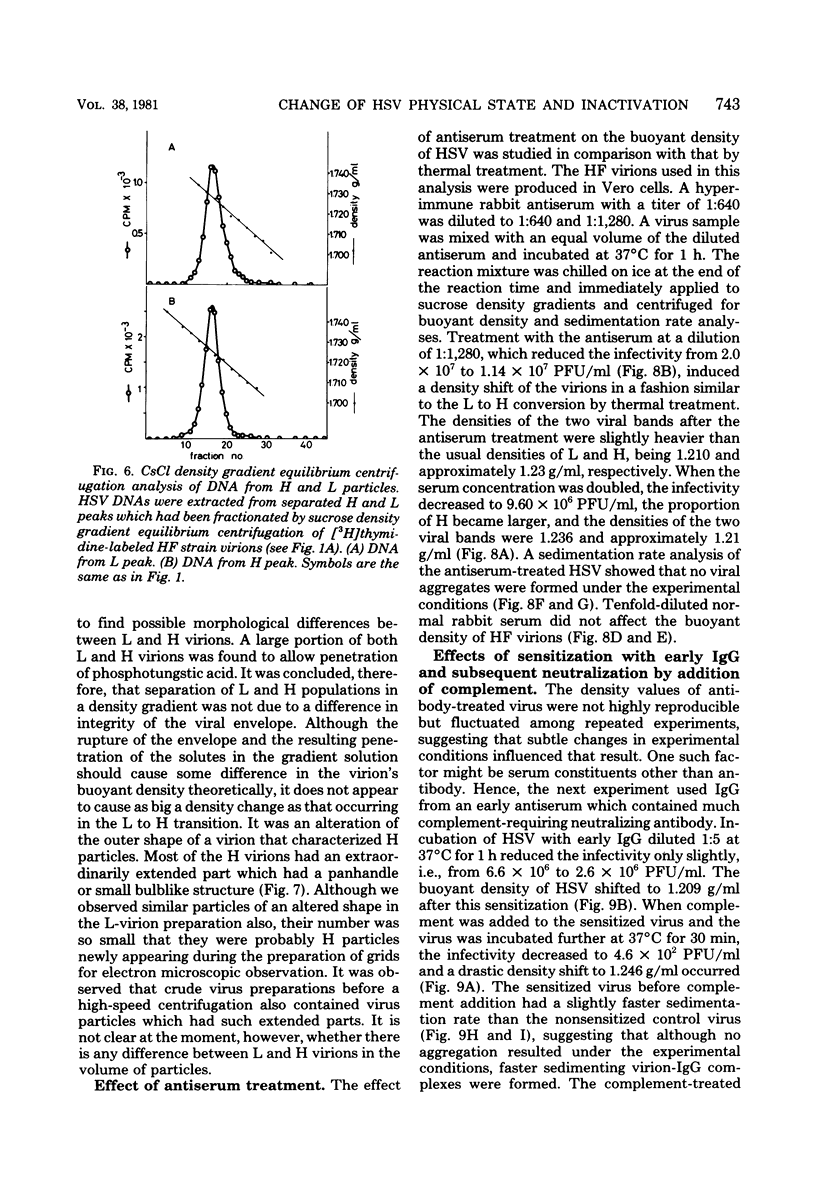
The buoyant density characteristics of infectious particles of herpes simplex virus types 1 and 2 were studied by centrifugation in sucrose and cesium chloride density gradients with a high resolution and satisfactory infectivity recovery. It was shown that two populations of infectious virions differing in buoyant density coexisted, the difference being slight but definite. The ratio of heavy (H) to light (L) viral particles varied depending upon the solute used, the strains of virus, and the cell origin. Circumstances favoring degradation of viral infectivity tended to increase the H portion. Incubation at 37 degrees C largely converted L to H, and heating at 45 degrees C converted all virions to H without infectivity. The L to H conversion was irreversible, and no populations intermediate between L and H were clearly observed. Inactivation by UV light irradiation did not affect the density pattern. That H was not an artefact due to penetration of solutes, osmotic pressure, viral aggregation, or loss of the envelope was shown experimentally. A difference in the outer shape of particles between negatively stained L and H populations was demonstrated by electron microscopy. Both cell-released and cell-bound herpes simplex virus particles gave essentially the same result with respect to the above characteristics. The effect of limiting dilutions of antiserum was similar to that of mild thermal treatment, in that denser virions increased parallel to a decrease in less dense virions. Sensitization with early immunoglobulin G, composed mainly of complement-requiring neutralizing antibody, caused the density transition, and subsequent addition of complement resulted in a further increase in the buoyant density of the sensitized virions. The DNA in virus particles neutralized with immunoglobulin G plus complement remained resistant to DNase treatment. Possible implications of the phenomena are discussed.
Full text
PDF


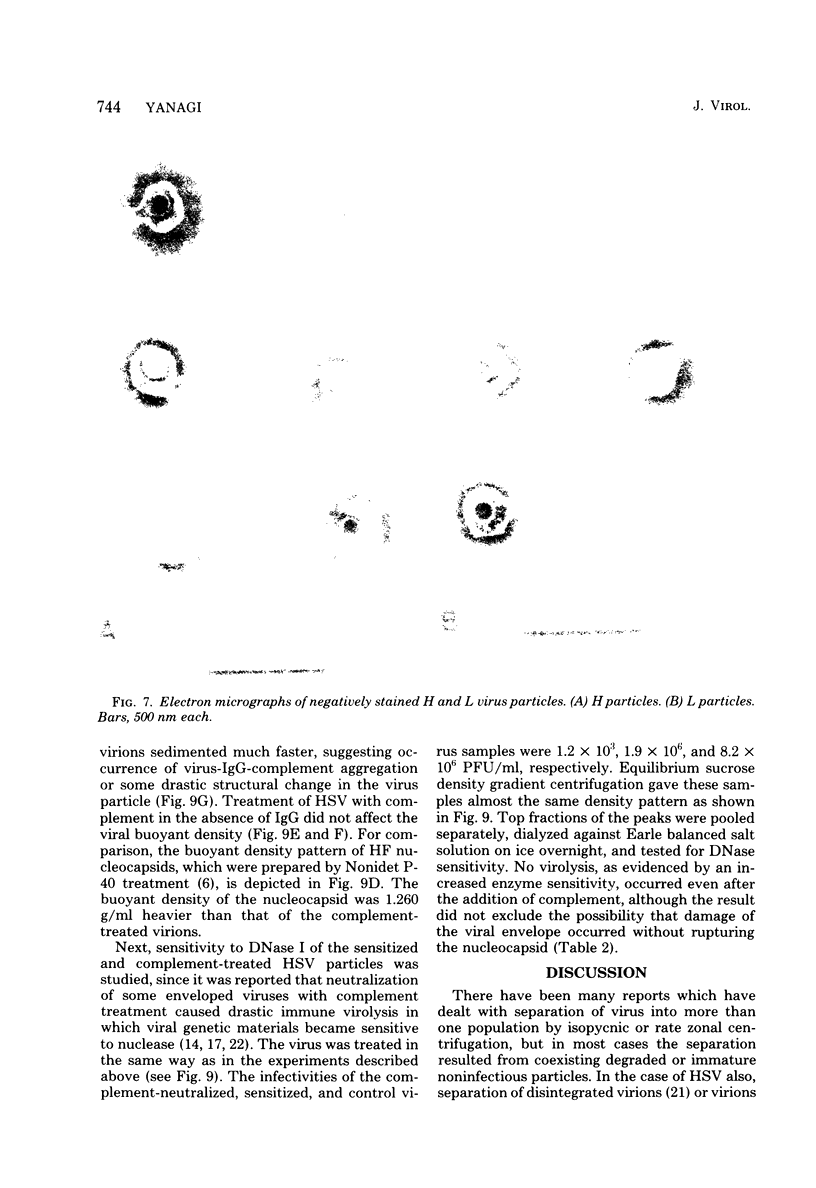
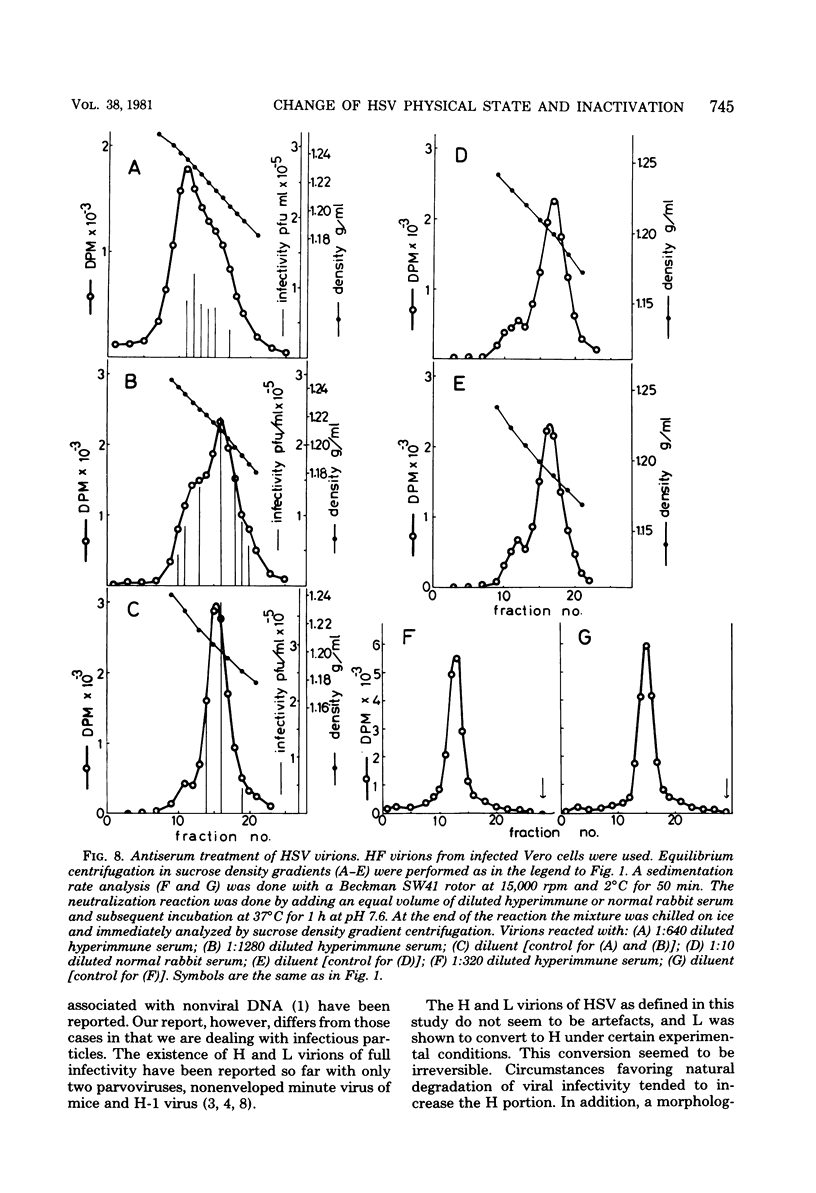
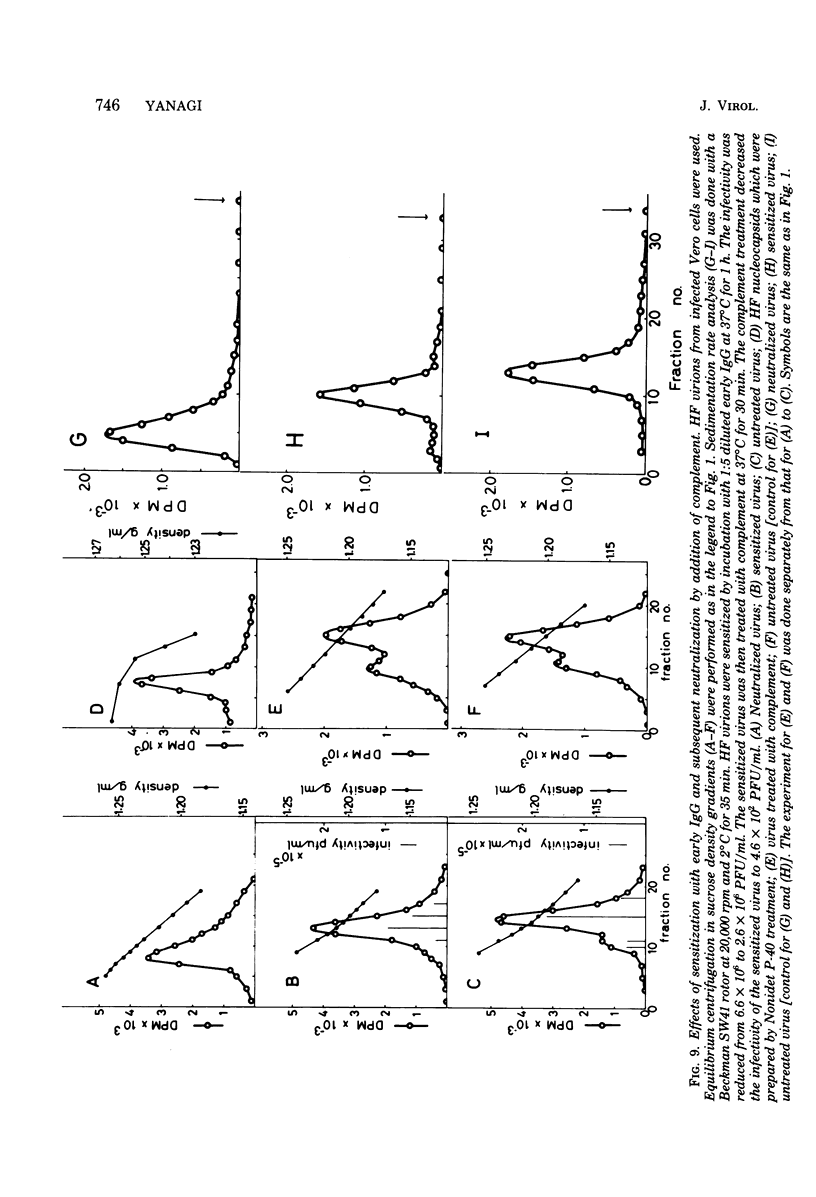


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurelian L., Wagner R. R. Two populations of herpes virus virions which appear to differ in physical properties and DNA composition. Proc Natl Acad Sci U S A. 1966 Sep;56(3):902–909. doi: 10.1073/pnas.56.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew P. The surface nature of proteins of a bovine enterovirus, before and after neutralization. J Gen Virol. 1976 Jul;32(1):17–23. doi: 10.1099/0022-1317-32-1-17. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Hayashi M. The parovivirus MVM: particles with altered structural proteins. Virology. 1975 Jul;66(1):261–261. doi: 10.1016/0042-6822(75)90196-8. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Hayashi M. The parvovirus MVM: a comparison of heavy and light particle infectivity and their density conversion in vitro. Virology. 1976 Oct 1;74(1):57–63. doi: 10.1016/0042-6822(76)90127-6. [DOI] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Roizman B. Proteins specified by herpes simplex virus. IX. Contiguity of host and viral proteins in the plasma membrane of infected cells. J Virol. 1973 May;11(5):810–813. doi: 10.1128/jvi.11.5.810-813.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsvik J. R., Hopkins M. S., Ellem K. A. Subfractionation of CsCl-purified H-1 parvovirus on metrizamide gradients. Virology. 1979 Jul 30;96(2):646–651. doi: 10.1016/0042-6822(79)90122-3. [DOI] [PubMed] [Google Scholar]
- Korant B. D., Lonberg-Holm K., Yin F. H., Noble-Harvey J. Fractionation of biologically active and inactive populations of human rhinovirus type 2. Virology. 1975 Feb;63(2):384–394. doi: 10.1016/0042-6822(75)90311-6. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C. Purification of equine herpesvirus type 1. J Gen Virol. 1976 Apr;31(1):81–91. doi: 10.1099/0022-1317-31-1-81. [DOI] [PubMed] [Google Scholar]
- Mandel B. Characterization of type 1 poliovirus by electrophoretic analysis. Virology. 1971 Jun;44(3):554–568. doi: 10.1016/0042-6822(71)90369-2. [DOI] [PubMed] [Google Scholar]
- Mandel B. Neutralization of animal viruses. Adv Virus Res. 1978;23:205–268. doi: 10.1016/S0065-3527(08)60101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matis J., Golaisová E. Influence of host cell type on the density of herpes simplex virus particles. Acta Virol. 1976 Dec;20(6):455–459. [PubMed] [Google Scholar]
- Oroszlan S., Gilden R. V. Immune virolysis: effect of antibody and complement on C-type RNA virus. Science. 1970 Jun 19;168(3938):1478–1480. doi: 10.1126/science.168.3938.1478. [DOI] [PubMed] [Google Scholar]
- Pertoft H., Bäck O., Lindahl-Kiessling K. Separation of various blood cells in colloidal silica-polyvinylpyrrolidone gradients. Exp Cell Res. 1968 May;50(2):355–368. doi: 10.1016/0014-4827(68)90454-0. [DOI] [PubMed] [Google Scholar]
- Pertoft H. Density gradient centrifugation of a herpesvirus (IBRV) in colloidal silica. Virology. 1970 Jun;41(2):368–372. doi: 10.1016/0042-6822(70)90090-5. [DOI] [PubMed] [Google Scholar]
- Radwan A. I., Burger D., Davis W. C. The fate of sensitized equine arteritis virus following neutralization by complement of anti-IgG serum. Virology. 1973 Jun;53(2):372–378. doi: 10.1016/0042-6822(73)90216-x. [DOI] [PubMed] [Google Scholar]
- Robinson D. J., Watson D. H. Structural proteins of herpes simplex virus. J Gen Virol. 1971 Feb;10(2):163–171. doi: 10.1099/0022-1317-10-2-163. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Buoyant density of herpes simplex virus in solutions of caesium chloride. Nature. 1967 May 13;214(5089):713–714. doi: 10.1038/214713a0. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring S. B., Roizman B. Herpes simplex virus products in productive and abortive infection. I. Stabilization with formaldehyde and preliminary analyses by isopycnic centrifugation in CsCl. J Virol. 1967 Apr;1(2):294–301. doi: 10.1128/jvi.1.2.294-301.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar V. Immune lysis of Sindbis virus. Virology. 1975 Aug;66(2):620–624. doi: 10.1016/0042-6822(75)90235-4. [DOI] [PubMed] [Google Scholar]
- TANIGUCHI S., YOSHINO K. AN ANALYSIS OF THE PLAQUE ASSAY OF HERPES SIMPLEX VIRUS IN CHICK EMBRYO MONOLAYERS. Arch Gesamte Virusforsch. 1964 Mar 13;14:537–552. doi: 10.1007/BF01555084. [DOI] [PubMed] [Google Scholar]
- TANIGUCHI S., YOSHINO K. STUDIES ON THE NEUTRALIZATION OF HERPES SIMPLEX VIRUS. II. ANALYSIS OF COMPLEMENT AS THE ANTIBODY-POTENTIATING FACTOR. Virology. 1965 May;26:54–60. doi: 10.1016/0042-6822(65)90025-5. [DOI] [PubMed] [Google Scholar]
- Tada A., Yoshino K. A new microplate neutralization test for typing of herpes simplex virus. Microbiol Immunol. 1978;22(7):415–426. doi: 10.1111/j.1348-0421.1978.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Yanagi K., Rush M. G., Biegeleisen K. Integration of herpes simplex virus type 1 DNA into the DNA of growth-arrested BHK-21 cells. J Gen Virol. 1979 Sep;44(3):657–667. doi: 10.1099/0022-1317-44-3-657. [DOI] [PubMed] [Google Scholar]
- Yanagi K., Talavera A., Nishimoto T., Rush M. G. Inhibition of herpes simplex virus type 1 replication in temperature-sensitive cell cycle mutants. J Virol. 1978 Jan;25(1):42–50. doi: 10.1128/jvi.25.1.42-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino K., Isono N. Studies on the neutralization of herpes simplex virus. IX. Variance in complement requirement among IgG and IgM from early and late sera under different sensitization conditions. Microbiol Immunol. 1978;22(7):403–414. doi: 10.1111/j.1348-0421.1978.tb00386.x. [DOI] [PubMed] [Google Scholar]



