Abstract
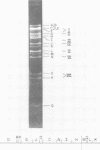
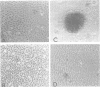
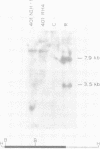
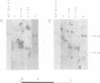
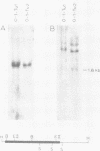
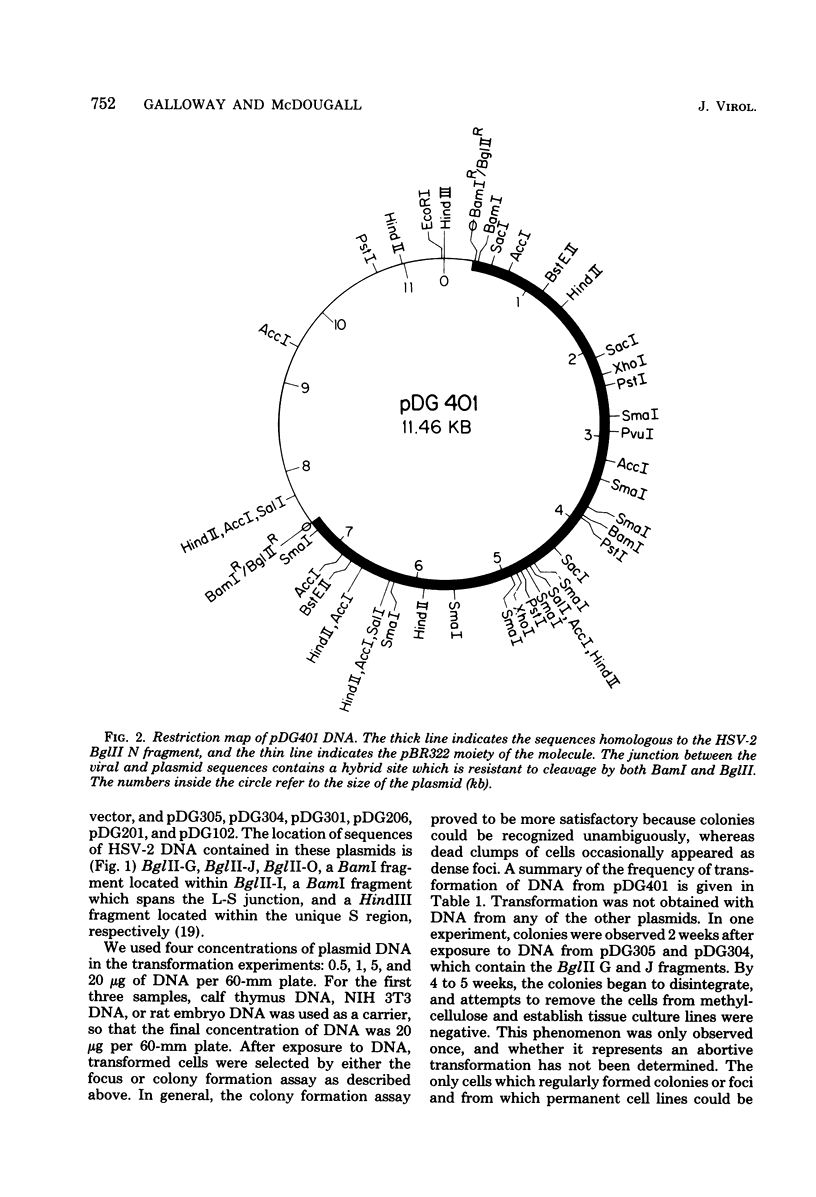
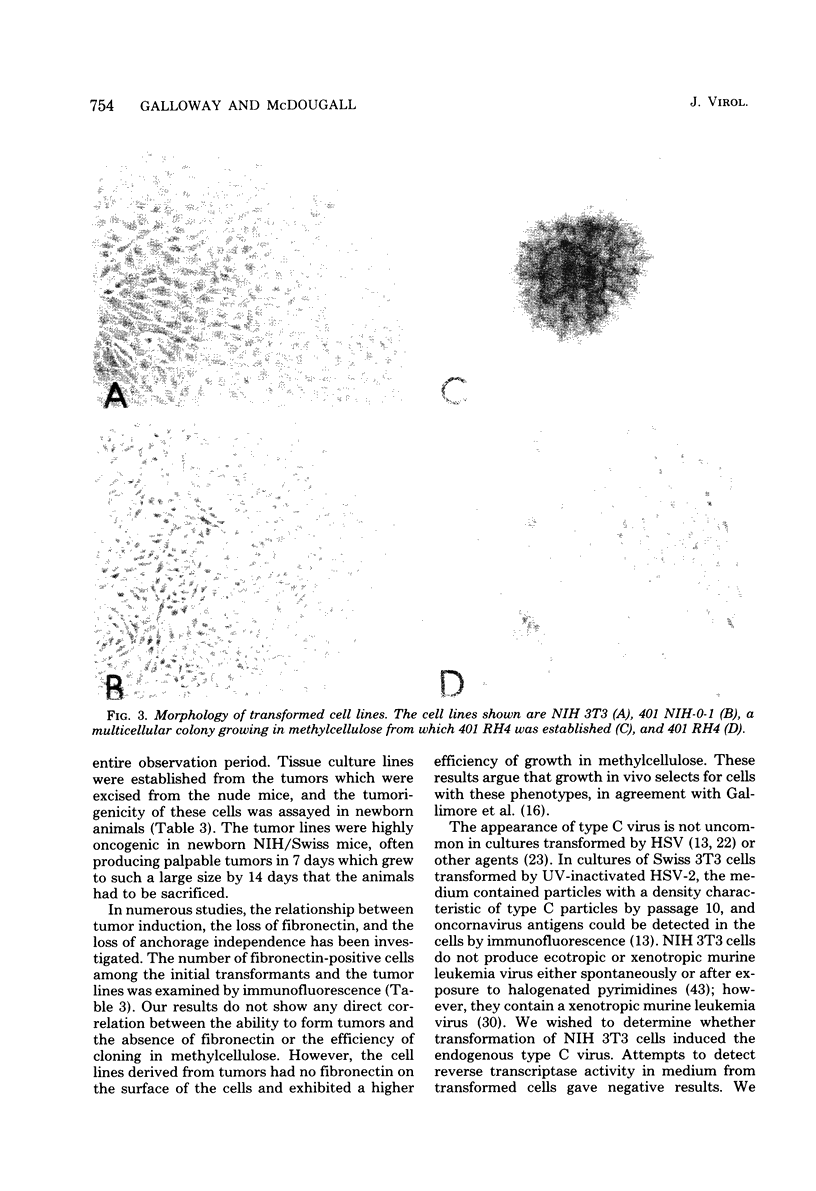
Transformation of rodent cells with isolated restriction endonuclease fragments of herpes simplex virus type 2 DNA identified a region of the genome located between map positions 0.58 and 0.62. These sequences were cloned into pBR322, and the recombinant plasmid was used to transform primary rat embryo cells and NIH 3T3 cells. The transformants were selected for their ability to form dense foci on a monolayer or to form colonies in semisolid medium. In contrast to the parental rat or mouse cells, cell lines transformed with the cloned herpes simplex virus type 2 fragment grow to high saturation densities, replicate in medium containing 1% serum, form colonies in dilute methylcellulose, show reduced levels of fibronectin, and are tumorigenic in nude mice and in their syngeneic hosts. Southern blot hybridizations have detected sequences homologous to the viral fragment in high-molecular-weight DNA from the transformed cell lines that are not present in DNA from normal rodents. In all cases, the plasmid DNA was present in less than one copy per cell, and the patterns of viral sequences changed with passage of the cell line in vivo.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Boyd A. L., Orme T. W. Transformation of mouse cells after infection with ultraviolet irradiation-inactivated herpes simplex virus type 2. Int J Cancer. 1975 Oct 15;16(4):526–538. doi: 10.1002/ijc.2910160403. [DOI] [PubMed] [Google Scholar]
- Camacho A., Spear G. Transformation of hamster embryo fibroblasts by a specific fragment of the herpes simplex virus genome. Cell. 1978 Nov;15(3):993–1002. doi: 10.1016/0092-8674(78)90283-0. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Gallimore P. H., McDougall J. K. Correlation between tumor induction and the large external transformation sensitive protein on the cell surface. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3570–3574. doi: 10.1073/pnas.73.10.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard W., Thornton H., Green M. Cells transformed by human Herpesvirus type 2 transcribe virus-specific RNA sequences shared by Herpesvirus types 1 and 2. Nat New Biol. 1973 Jun 27;243(130):264–266. doi: 10.1038/newbio243264a0. [DOI] [PubMed] [Google Scholar]
- Copple C. D., McDougall J. K. Clonal derivatives of a herpes type 2 transformed hamster cell line (333-8-9): cytogenetic analysis, tumorigenicity and virus sequence detection. Int J Cancer. 1976 Apr 15;17(4):501–510. doi: 10.1002/ijc.2910170413. [DOI] [PubMed] [Google Scholar]
- Darai G., Munk K. Human embryonic lung cells abortively infected with Herpes virus hominis type 2 show some properties of cell transformation. Nat New Biol. 1973 Feb 28;241(113):268–269. doi: 10.1038/newbio241268a0. [DOI] [PubMed] [Google Scholar]
- Davis D. B., Kingsbury D. T. Quantitation of the viral DNA present in cells transformed by UV-irradiated herpes simplex virus. J Virol. 1976 Mar;17(3):788–793. doi: 10.1128/jvi.17.3.788-793.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster cells after exposure to herpes simplex virus type 2. Nat New Biol. 1971 Sep 8;233(36):48–50. doi: 10.1038/newbio233048a0. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster embryo cells after exposure to inactivated herpes simplex virus type 1. J Virol. 1973 Aug;12(2):209–217. doi: 10.1128/jvi.12.2.209-217.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff R., Rapp F. Quantitative assay for transformation of 3T3 cells by herpes simplex virus type 2. J Virol. 1975 Mar;15(3):490–496. doi: 10.1128/jvi.15.3.490-496.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery V. L., Courtney R. J., Schaffer P. A. Expression of an early, nonstructural antigen of herpes simplex virus in cell transformed in vitro by herpes simplex virus. J Virol. 1977 Jan;21(1):284–291. doi: 10.1128/jvi.21.1.284-291.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel N., Locker H., Cox B., Roizman B., Rapp F. Herpes simplex virus DNA in transformed cells: sequence complexity in five hamster cell lines and one derived hamster tumor. J Virol. 1976 Jun;18(3):885–893. doi: 10.1128/jvi.18.3.885-893.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore P. H., McDougall J. K., Chen L. B. In vitro traits of adenovirus-transformed cell lines and their relevance to tumorigenicity in nude mice. Cell. 1977 Apr;10(4):669–678. doi: 10.1016/0092-8674(77)90100-3. [DOI] [PubMed] [Google Scholar]
- Galloway D. A., Copple C. D., McDougall J. K. Analysis of viral DNA sequences in hamster cells transformed by herpes simplex virus type 2. Proc Natl Acad Sci U S A. 1980 Feb;77(2):880–884. doi: 10.1073/pnas.77.2.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., Lukanidin E., Topp W. C., Sambrook J. Transformation of rat cells by the hybrid virus Ad2(2+) HEY. J Gen Virol. 1979 Feb;42(2):339–356. doi: 10.1099/0022-1317-42-2-339. [DOI] [PubMed] [Google Scholar]
- Galloway D. A., Swain M. Cloning of Herpes simplex virus 2 DNA fragments in a plasmid vector. Gene. 1980 Nov;11(3-4):253–257. doi: 10.1016/0378-1119(80)90065-7. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guerry P., Falkow S. Polynucleotide sequence relationships among some bacterial plasmids. J Bacteriol. 1971 Jul;107(1):372–374. doi: 10.1128/jb.107.1.372-374.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Aaronson S. A., Derge J. G., Chakrabarty M., Showalter S. D., Dunn C. Y. Activation of an endogenous mouse type C virus by ultraviolet-irradiated herpes simplex virus types 1 and 2. Proc Natl Acad Sci U S A. 1976 Feb;73(2):646–650. doi: 10.1073/pnas.73.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Boyd A. L., Derge J. G., Zweig M., Eader L., Showalter S. D. Comparison of properties of mouse cells transformed spontaneously by ultraviolet light-irradiated herpes simplex virus or by simian virus 40. Cancer Res. 1980 Jul;40(7):2213–2222. [PubMed] [Google Scholar]
- Hayward G. S., Jacob R. J., Wadsworth S. C., Roizman B. Anatomy of herpes simplex virus DNA: evidence for four populations of molecules that differ in the relative orientations of their long and short components. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4243–4247. doi: 10.1073/pnas.72.11.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariwalla R. J., Aurelian L., Ts'o P. O. Tumorigenic transformation induced by a specific fragment of DNA from herpes simplex virus type 2. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2279–2283. doi: 10.1073/pnas.77.4.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessous A., Bibor-Hardy V., Suh M., Simard R. Analysis of chromosomes, nucleic acids, and polypeptides in hamster cells transformed by herpes simplex virus type 2. Cancer Res. 1979 Aug;39(8):3225–3234. [PubMed] [Google Scholar]
- Kimura S., Flannery V. L., Levy B., Schaffer P. A. Oncogenic transformation of primary hamster cells by herpes simplex virus type 2 (hsv-2) and an hsv-2 temperature-sensitive mutant. Int J Cancer. 1975 May 15;15(5):786–798. doi: 10.1002/ijc.2910150510. [DOI] [PubMed] [Google Scholar]
- Kucera L. S., Gusdon J. P. Transformation of human embryonic fibroblasts by photodynamically inactivated herpes simplex virus, type 2 at supra-optimal temperature. J Gen Virol. 1976 Feb;30(2):257–261. doi: 10.1099/0022-1317-30-2-257. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- Macnab J. C. Transformation of rat embryo cells by temperature-sensitive mutants of herpes simplex virus. J Gen Virol. 1974 Jul;24(1):143–153. doi: 10.1099/0022-1317-24-1-143. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- McDougall J. K., Masse T. H., Galloway D. A. Location and cloning of the herpes simplex virus type 2 thymidine kinase gene. J Virol. 1980 Mar;33(3):1221–1224. doi: 10.1128/jvi.33.3.1221-1224.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson A. C., Thouless M. E., Eglin R. P., Darby G. The detection of virus DNA sequences in a herpes type 2 transformed hamster cell line (333-8-9). Int J Cancer. 1976 Apr 15;17(4):493–500. doi: 10.1002/ijc.2910170412. [DOI] [PubMed] [Google Scholar]
- Nachtigal M., Duff R., Rapp F. Chromosome aberrations in Syrian hamster embryo cells transformed after exposure to ultraviolet-irradiated herpes simplex virus type 1 or 2. J Natl Cancer Inst. 1975 Jan;54(1):97–105. doi: 10.1093/jnci/54.1.97. [DOI] [PubMed] [Google Scholar]
- Rapp F., Li J. L., Jerkofsky M. Transformation of mammalian cells by DNA-containing viruses following photodynamic inactivation. Virology. 1973 Oct;55(2):339–346. doi: 10.1016/0042-6822(73)90173-6. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Bacchetti S., Graham F. L. Relation of Herpes simplex viruses to human malignancies. Curr Top Microbiol Immunol. 1977;77:71–95. doi: 10.1007/978-3-642-66740-4_3. [DOI] [PubMed] [Google Scholar]
- Reyes G. R., LaFemina R., Hayward S. D., Hayward G. S. Morphological transformation by DNA fragments of human herpesviruses: evidence for two distinct transforming regions in herpes simplex virus types 1 and 2 and lack of correlation with biochemical transfer of the thymidine kinase gene. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):629–641. doi: 10.1101/sqb.1980.044.01.066. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stephension J. R., Reynolds R. K., Tronick S. R., Aaronson S. A. Distribution of three classes of endogenous type-C RNA viruses among inbred strains of mice. Virology. 1975 Oct;67(2):404–414. doi: 10.1016/0042-6822(75)90442-0. [DOI] [PubMed] [Google Scholar]
- Suh M., Kessous A., Poirier N., Simard R. Immunoprecipitation of polypeptides from hamster embryo cells transformed by herpes simplex virus type 2. Virology. 1980 Jul 30;104(2):303–311. doi: 10.1016/0042-6822(80)90335-9. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Yamanishi K. Transformation of hamster embryo and human embryo cells by temperature sensitive mutants of herpes simplex virus type 2. Virology. 1974 Sep;61(1):306–311. doi: 10.1016/0042-6822(74)90267-0. [DOI] [PubMed] [Google Scholar]
- Van der Eb A. J., Mulder C., Graham F. L., Houweling A. Transformation with specific fragments of adenovirus DNAs. I. Isolation of specific fragments with transforming activity of adenovirus 2 and 5 DNA. Gene. 1977;2(3-4):115–132. doi: 10.1016/0378-1119(77)90012-9. [DOI] [PubMed] [Google Scholar]
- Walboomers J. M., Schegget J. T. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976 Oct 1;74(1):256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Macnab J. C., Subak-Sharpe J. H. The structure and biological properties of herpes simplex virus DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):657–666. doi: 10.1101/sqb.1974.039.01.079. [DOI] [PubMed] [Google Scholar]