Abstract
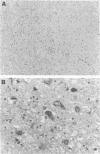
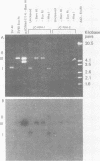
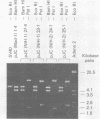
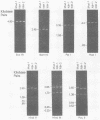
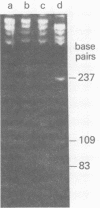
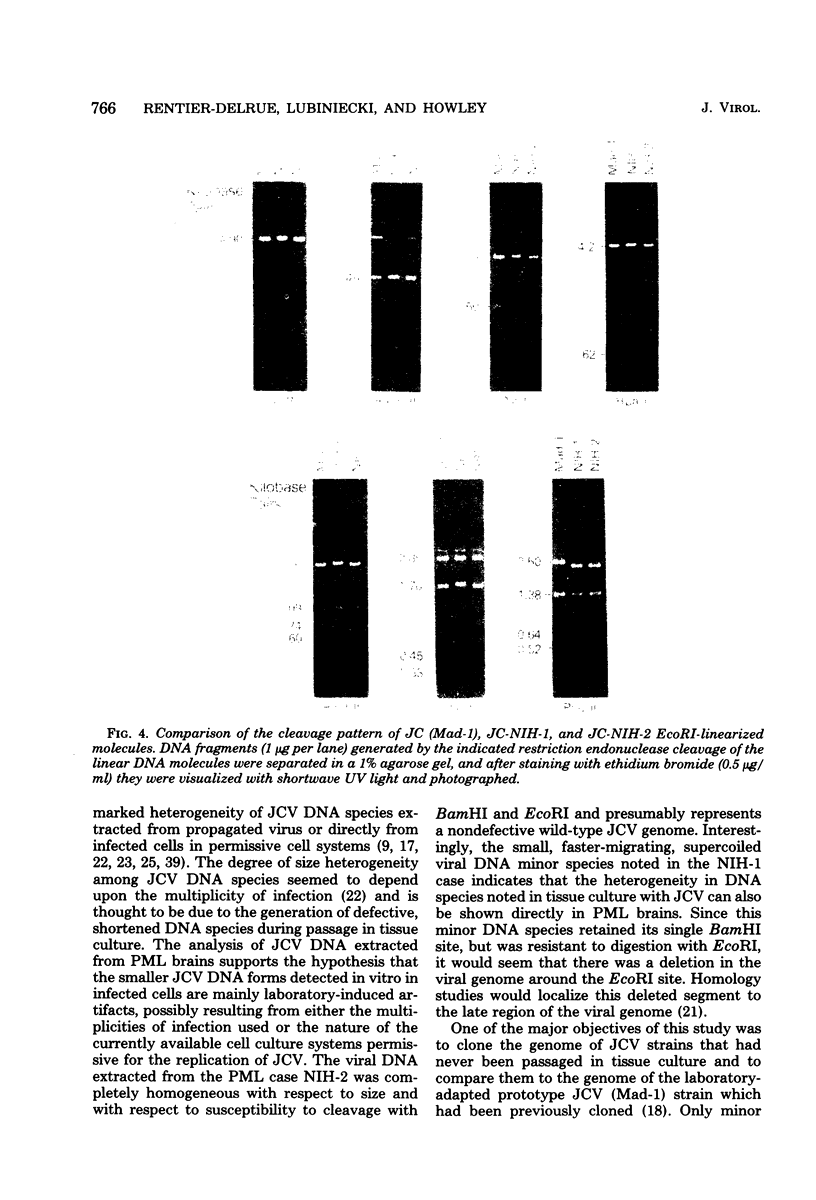
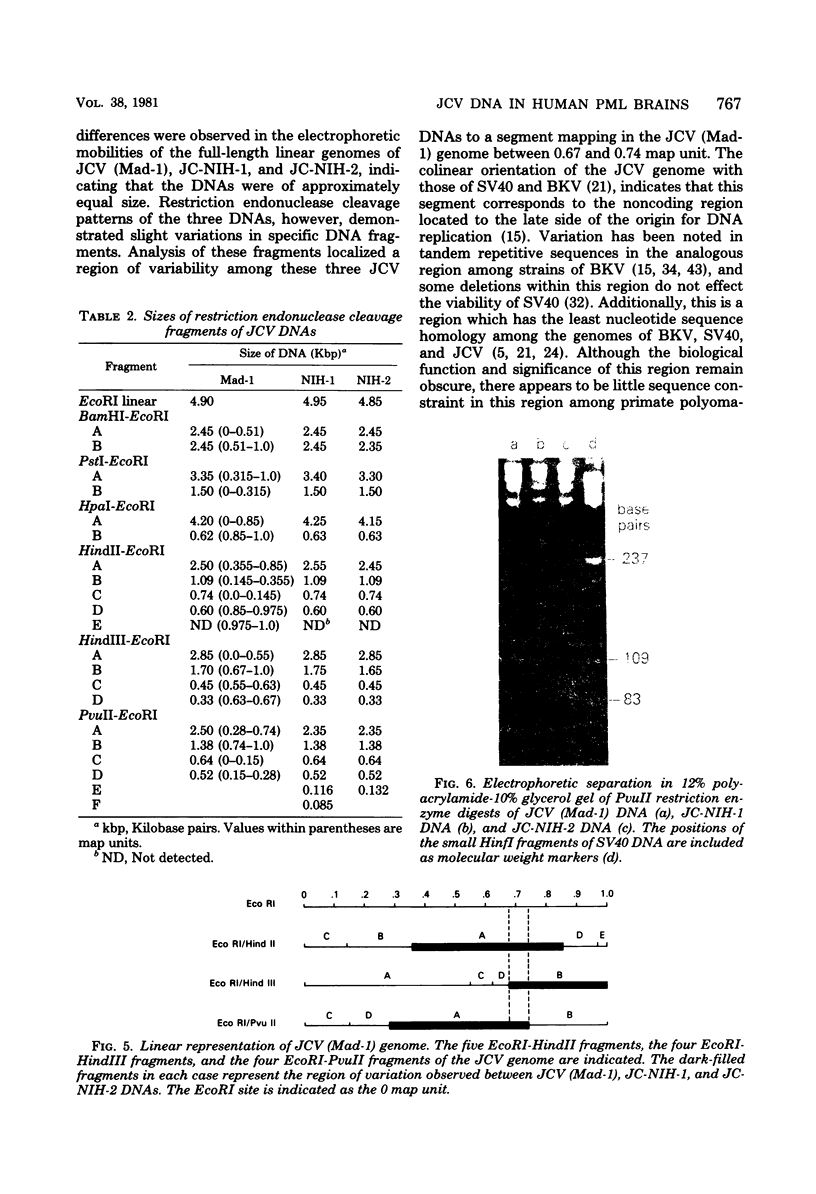
Human polyomavirus JC DNA was purified directly from the diseased brain tissue of two patients with progressive multifocal leukoencephalopathy (PML) by a method employing differential salt precipitation (B. Hirt, J. Mol. Biol. 26:365-369, 1967). Each of the viral genomes (JC-NIH-1 and JC-NIH-2) was molecularly cloned intact in Escherichia coli, using pBR322, at their unique EcoRI (0.00 map unit) and BamHI (0.51 map unit) sites. The JC-NIH-1 genome was approximately 50 base pairs larger and the JC-NIH-2 genome was approximately 50 base pairs smaller than the prototype human polyomavirus JC (Mad-1) DNA. Analysis of the restriction endonuclease cleavage fragments of these two DNAs and the human polyomavirus JC (Mad-1) DNA revealed only slight differences which mapped in a region of the genome extending from 0.67 to 0.74 map unit. From previous homology studies, this region of variance corresponds to the noncoding region to the late side of the origin of DNA replication.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. V., Daniel R. A., Gardner S. D., Field A. M., Gibson P. E. Polyoma virus in urine during pregnancy. Lancet. 1977 Oct 1;2(8040):709–710. doi: 10.1016/s0140-6736(77)90514-1. [DOI] [PubMed] [Google Scholar]
- Dhar R., Lai C. J., Khoury G. Nucleotide sequence of the DNA replication origin for human papovavirus BKV: sequence and structural homology with SV40. Cell. 1978 Feb;13(2):345–358. doi: 10.1016/0092-8674(78)90203-9. [DOI] [PubMed] [Google Scholar]
- Dörries K., Johnson R. T., ter Meulen V. Detection of polyoma virus DNA in PML-brain tissue by (in situ) hybridization. J Gen Virol. 1979 Jan;42(1):49–57. doi: 10.1099/0022-1317-42-1-49. [DOI] [PubMed] [Google Scholar]
- Freund J., di Mayorca G., Subramanian K. N. Mapping and ordering of fragments of BK virus DNA produced by restriction endonucleases. J Virol. 1979 Mar;29(3):915–925. doi: 10.1128/jvi.29.3.915-925.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J., Martin J. D., Padgett B. L., Walker D. L. Infectivity of the DNA from four isolates of JC virus. J Virol. 1979 Nov;32(2):476–482. doi: 10.1128/jvi.32.2.476-482.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hogan T. F., Borden E. C., McBain J. A., Padgett B. L., Walker D. L. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann Intern Med. 1980 Mar;92(3):373–378. doi: 10.7326/0003-4819-92-3-373. [DOI] [PubMed] [Google Scholar]
- Hogan T. F., Padgett B. L., Walker D. L., Borden E. C., McBain J. A. Rapid detection and identification of JC virus and BK virus in human urine by using immunofluorescence microscopy. J Clin Microbiol. 1980 Feb;11(2):178–183. doi: 10.1128/jcm.11.2.178-183.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley P. M., Israel M. A., Law M. F., Martin M. A. A rapid method for detecting and mapping homology between heterologous DNAs. Evaluation of polyomavirus genomes. J Biol Chem. 1979 Jun 10;254(11):4876–4883. [PubMed] [Google Scholar]
- Howley P. M., Khoury G., Takemoto K. K., Martin M. A. Polynucleotide sequences common to the genomes of simian virus 40 and the human papovaviruses JC and BK. Virology. 1976 Aug;73(1):303–307. doi: 10.1016/0042-6822(76)90084-2. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Rentier-Delrue F., Heilman C. A., Law M. F., Chowdhury K., Israel M. A., Takemoto K. K. Cloned human polyomavirus JC DNA can transform human amnion cells. J Virol. 1980 Dec;36(3):878–882. doi: 10.1128/jvi.36.3.878-882.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel M. A., Chan H. W., Rowe W. P., Martin M. A. Molecular cloning of polyoma virus DNA in Escherichia coli: plasmid vector system. Science. 1979 Mar 2;203(4383):883–887. doi: 10.1126/science.217087. [DOI] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Martin J. D., Takemoto K. K., Howley P. M. The colinear alignment of the genomes of papovaviruses JC, BK, and SV40. Virology. 1979 Jul 30;96(2):576–587. doi: 10.1016/0042-6822(79)90113-2. [DOI] [PubMed] [Google Scholar]
- Martin J. D., Frisque R. J., Padgett B. L., Walker D. L. Restriction endonuclease cleavage map of the DNA of JC virus. J Virol. 1979 Mar;29(3):846–855. doi: 10.1128/jvi.29.3.846-855.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura T., Yoshiike K., Takemoto K. K. Characterization of JC papovavirus adapted to growth in human embryonic kidney cells. J Virol. 1980 Aug;35(2):498–504. doi: 10.1128/jvi.35.2.498-504.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell N., Lai C. J., Khoury G., Kelly T. J., Jr Electron microscope study of the base sequence homology between simian virus 40 and human papovavirus BK. J Virol. 1978 Jan;25(1):193–201. doi: 10.1128/jvi.25.1.193-201.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn J. E., Robertson S. M., Padgett B. L., ZuRhein G. M., Walker D. L., Weisblum B. Comparison of JC and BK human papovaviruses with simian virus 40: restriction endonuclease digestion and gel electrophoresis of resultant fragments. J Virol. 1974 Mar;13(3):614–622. doi: 10.1128/jvi.13.3.614-622.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Rogers C. M., Walker D. L. JC virus, a human polyomavirus associated with progressive multifocal leukoencephalopathy: additional biological characteristics and antigenic relationships. Infect Immun. 1977 Feb;15(2):656–662. doi: 10.1128/iai.15.2.656-662.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. New human papovaviruses. Prog Med Virol. 1976;22:1–35. [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973 Apr;127(4):467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shah K. V., Ozer H. L., Ghazey H. N., Kelly T. J., Jr Common structural antigen of papovaviruses of the simian virus 40-polyoma subgroup. J Virol. 1977 Jan;21(1):179–186. doi: 10.1128/jvi.21.1.179-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Zain B. S., Roberts R. J., Weissman S. M. Mapping of the HhaI and HinfI cleavage sites on simian virus 40 DNA. J Mol Biol. 1977 Feb 25;110(2):297–317. doi: 10.1016/s0022-2836(77)80074-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Howley P. M., Miyamura T. JC human papovavirus replication in human amnion cells. J Virol. 1979 Apr;30(1):384–389. doi: 10.1128/jvi.30.1.384-389.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewska Z., Wellish M., Gilden D. Growth of JC virus in adult human brain cell cultures. Arch Virol. 1980;65(2):141–148. doi: 10.1007/BF01317325. [DOI] [PubMed] [Google Scholar]
- Yang R. C., Wu R. BK virus DNA: complete nucleotide sequence of a human tumor virus. Science. 1979 Oct 26;206(4417):456–462. doi: 10.1126/science.228391. [DOI] [PubMed] [Google Scholar]