Abstract
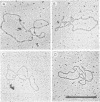
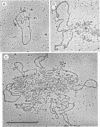
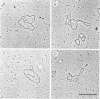
Electron microscopy was used to identify and quantitate DNA molecules associated with 3H-labeled polyoma minichromosomes which had been fractionated on a sucrose gradient. The percentage of replicating DNA molecules observed in the fractions of the gradient normally designated the replicative intermediate region was up to ninefold higher than in fractions from the mature region. Nevertheless, because of the higher overall concentration of polyoma DNA molecules in the mature region, nearly as many replicating DNA molecules were computed to be in the mature region as in the replicative intermediate region. The replicating molecules in the mature region was predominantly early replicative intermediates. Almost all late replicative intermediates were found in the replicative intermediate region. Under aqueous spreading conditions, a substantial fraction of the replicating DNA structures appeared to be asymmetrical or otherwise unusual, suggesting that extensive single-stranded regions may exist in replicating polyoma minichromosomes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldari C. T., Amaldi F., Buongiorno-Nardelli M. Electron microscopic analysis of replicating DNA of sea urchin embryos. Cell. 1978 Nov;15(3):1095–1107. doi: 10.1016/0092-8674(78)90293-3. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Ford C. C. Cytoplasmic control of nuclear DNA synthesis during early development of Xenopus laevis: a cell-free assay. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2437–2441. doi: 10.1073/pnas.72.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbow R. M., Krauss M. R. Recombinant DNA formation in a cell-free system from Xenopus laevis eggs. Cell. 1977 Sep;12(1):191–204. doi: 10.1016/0092-8674(77)90197-0. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Krauss M. R., Reeder R. H. DNA synthesis in a multi-enzyme system from Xenopus laevis eggs. Cell. 1978 Feb;13(2):307–318. doi: 10.1016/0092-8674(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Botchan P., Reeder R. H., Dawid I. B. Restriction analysis of the nontranscribed spacers of Xenopus laevis ribosomal DNA. Cell. 1977 Jul;11(3):599–607. doi: 10.1016/0092-8674(77)90077-0. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D. A symmetrical model for polyoma virus DNA replication. J Mol Biol. 1971 Dec 28;62(3):513–524. doi: 10.1016/0022-2836(71)90152-5. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Anderson S., Bar-Shavit R., Collins E., Edenberg H., Herman T., Karas B., Kaufmann G., Krokan H., Shelton E. Replication and structure of simian virus 40 chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):679–692. doi: 10.1101/sqb.1979.043.01.076. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Anderson S., DePamphilis M. L. Involvement of DNA polymerase alpha in simian virus 40 DNA replication. J Biol Chem. 1978 May 10;253(9):3273–3280. [PubMed] [Google Scholar]
- Fareed G. C., Garon G. F., Salzman N. P. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):484–491. doi: 10.1128/jvi.10.3.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlie B. B., Krauss M. R., Buckler-White A. J., Benbow R. M., Pigiet V. Polyoma virus minichromosomes: a soluble in vitro replication system. J Virol. 1981 Jun;38(3):805–814. doi: 10.1128/jvi.38.3.805-814.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlie B. B., Pigiet V., Breaux C. B., Krauss M. R., King C. R., Benbow R. M. Polyoma virus minichromosomes: associated enzyme activities. J Virol. 1981 Jun;38(3):826–832. doi: 10.1128/jvi.38.3.826-832.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Replicating molecules of polyoma virus DNA. J Mol Biol. 1969 Feb 28;40(1):141–144. doi: 10.1016/0022-2836(69)90302-7. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Robberson D. L., Vinograd J. A novel closed-circular mitochondrial DNA with properties of a replicating intermediate. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2252–2257. doi: 10.1073/pnas.68.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B., Fanning E., Richter A. DNA polymerases and a single-strand-specific DNA-binding protein associated with simian virus 40 nucleoprotein complexes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):705–708. doi: 10.1101/sqb.1979.043.01.078. [DOI] [PubMed] [Google Scholar]
- Raleigh E. A., Davis R. W. Determination of DNA concentration by electron microscopy. Anal Biochem. 1976 May 7;72:460–467. doi: 10.1016/0003-2697(76)90554-6. [DOI] [PubMed] [Google Scholar]
- Su R. T., DePamphilis M. L. In vitro replication of simian virus 40 DNA in a nucleoprotein complex. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3466–3470. doi: 10.1073/pnas.73.10.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota Y., Waqar M. A., Burke J. F., Milavetz B. I., Evans M. J., Kowalski D., Huberman J. A. Association of enzymes with replicating and nonreplicating simian virus 40 chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):693–704. doi: 10.1101/sqb.1979.043.01.077. [DOI] [PubMed] [Google Scholar]
- Waldeck W., Spaeren U., Mastromei G., Eliasson R., Reichard P. Replication of polyoma DNA in nuclear extracts and nucleoprotein complexes. J Mol Biol. 1979 Dec 15;135(3):675–689. doi: 10.1016/0022-2836(79)90171-2. [DOI] [PubMed] [Google Scholar]