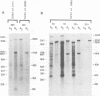
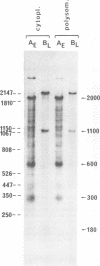
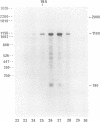
Abstract
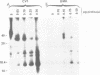
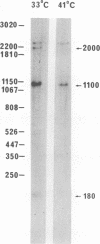
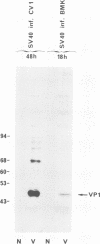
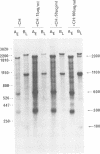
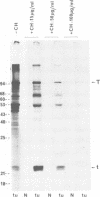
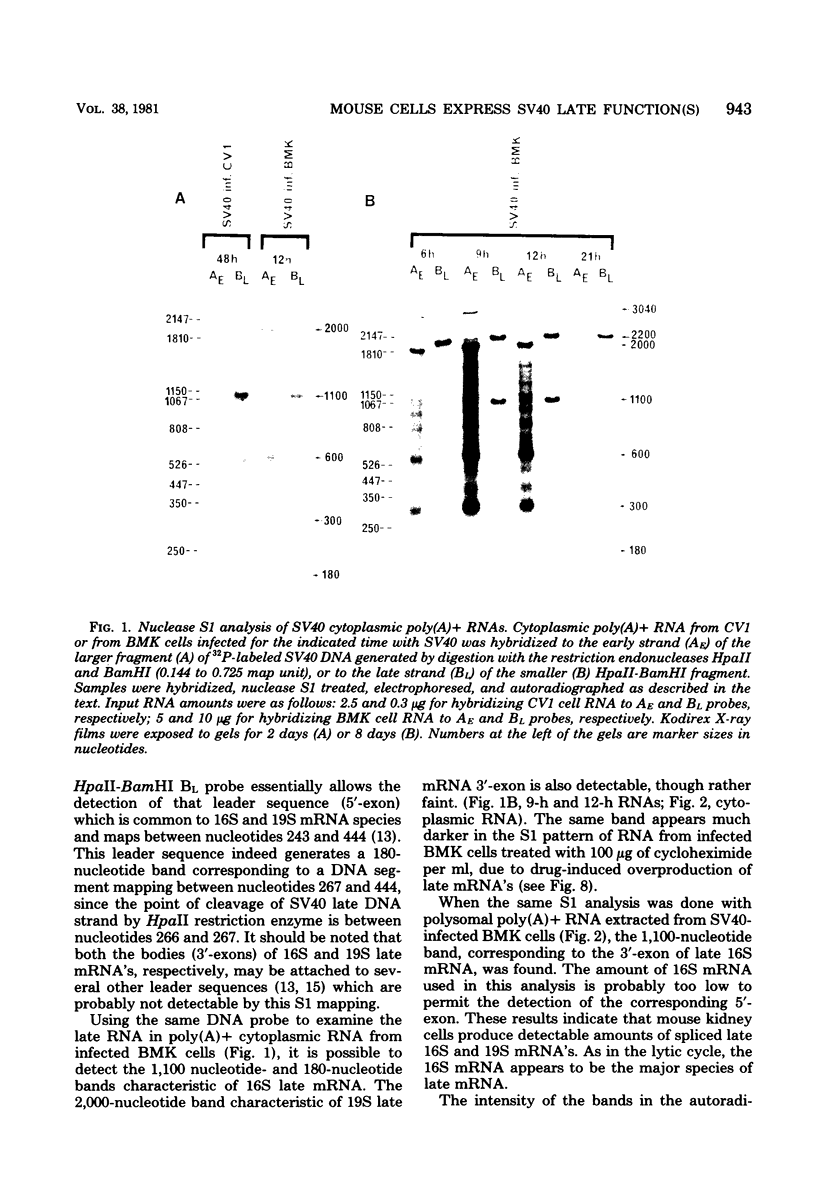
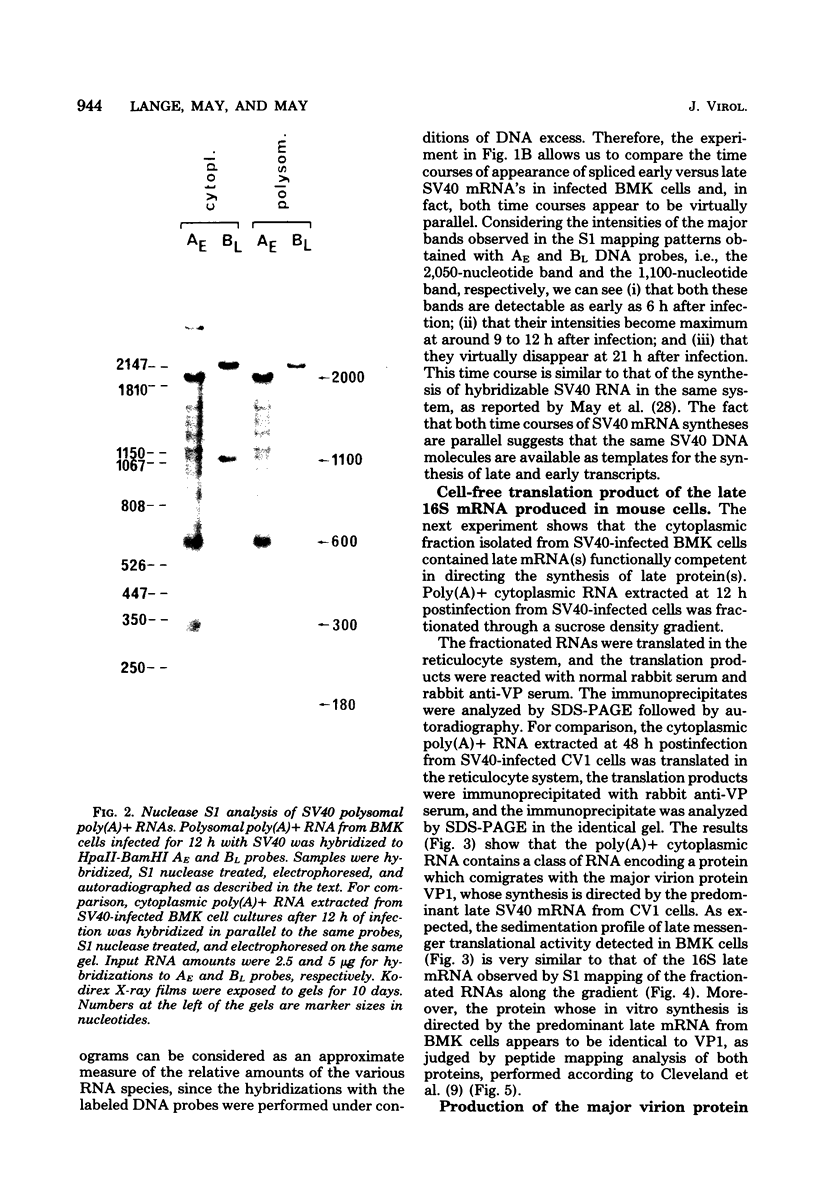
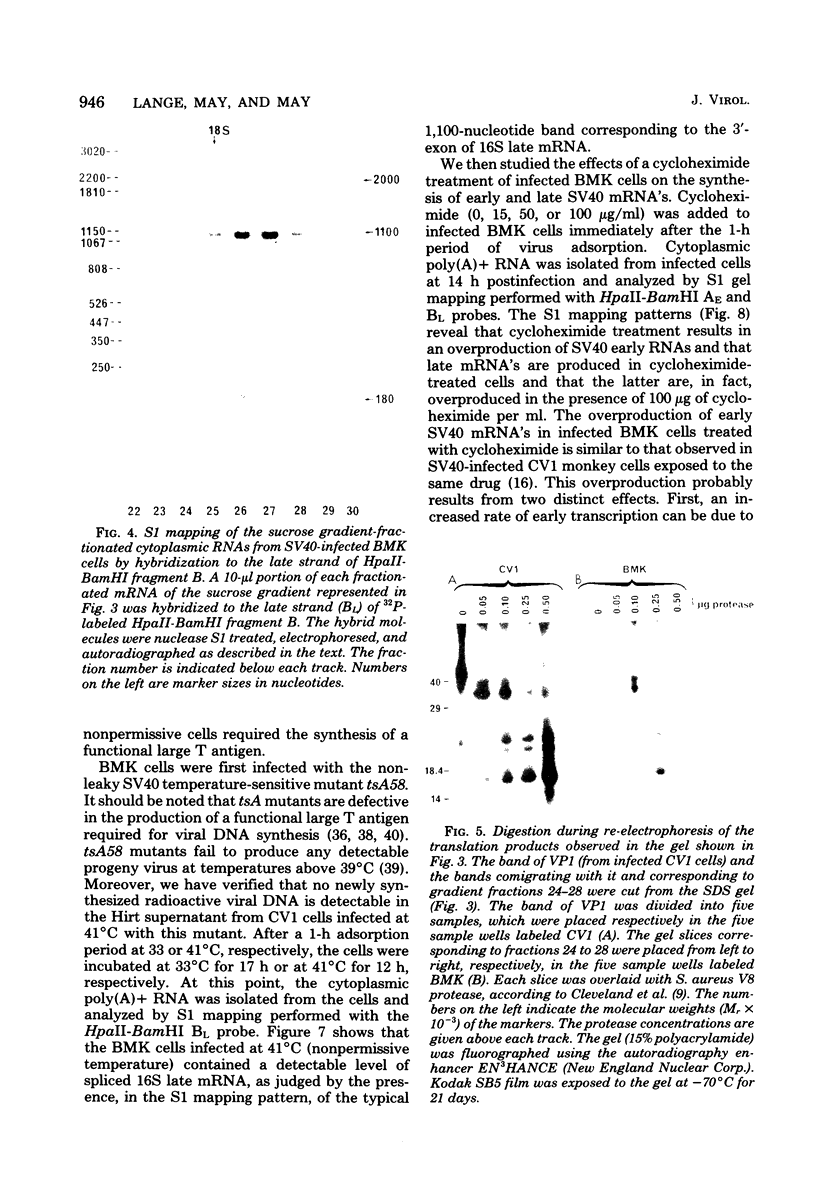
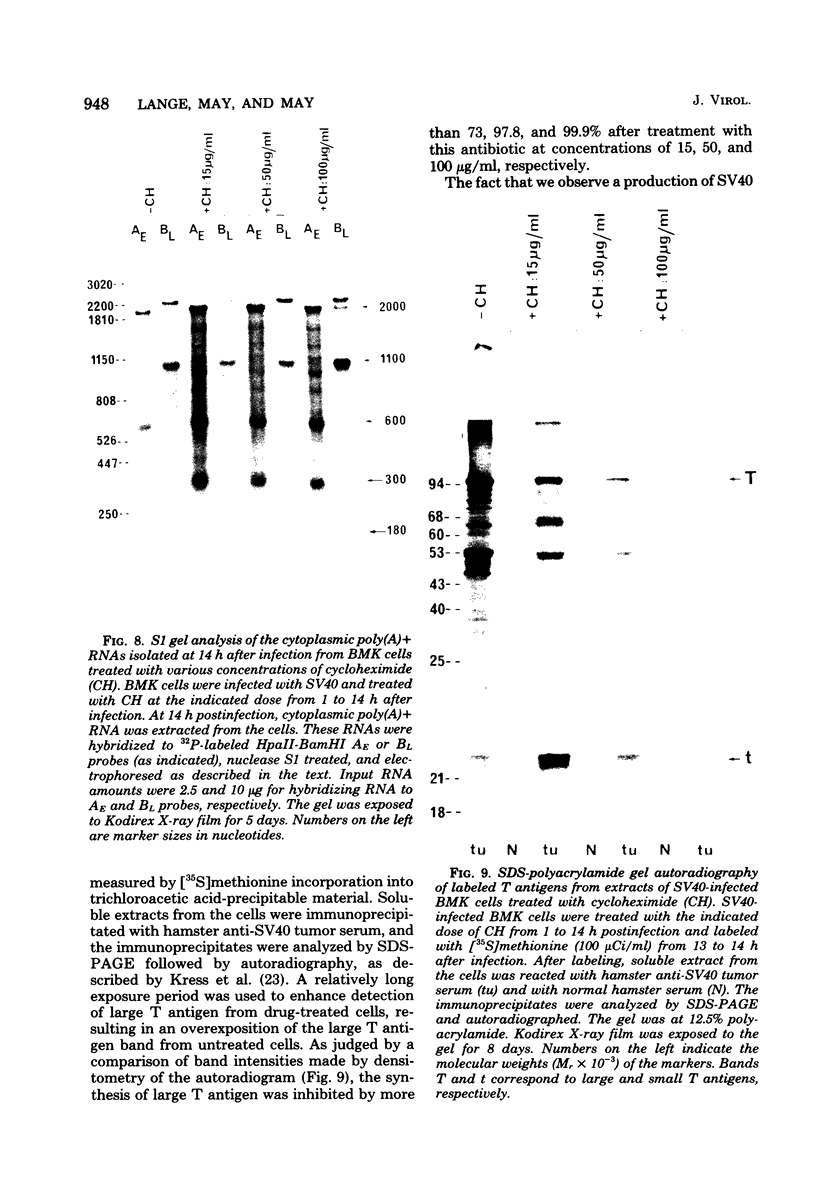
Mouse cells are fully nonpermissive for simian virus 40 (SV40). Infection does not lead to detectable virus replication. In this report, it was shown, first, that spliced 16S and 19S SV40 late mRNA were present in cytoplasmic and polysomal polyadenylated acid+ RNA preparations from SV40-infected baby mouse kidney cells. The 16S and 19S SV40 late mRNA's produced in infected baby mouse kidney cells were identical to or similar to the 16S and 19S SV40 late mRNA's produced in permissive monkey cells as judged by their S1 mapping patterns performed with the late strand of HpaII-BamHI fragment B and by their sedimentation patterns in a sucrose gradient. It was also shown that the 16S late mRNA from infected baby mouse kidney cells could be translated into a polypeptide which was identical to or similar to virion protein VP1 in every aspect examined, including the patter of peptide mapping by limited proteolysis. Second, we reported that mouse kidney cells produced detectable, although low, levels of SV40 virion protein VP1, as shown by the sodium dodecyl sulfate-polyacrylamide gel autoradiogram of [35S]methionine-labeled proteins immunoprecipitated by a rabbit antiserum directed against SV40 virion proteins. Third, it was reported that infected baby mouse kidney cells produced late mRNA's either (i) when the infection was done at a restrictive temperature with the nonleaky tsA58 mutant or (ii) in cells treated with 100 microgram of cycloheximide per ml, in which large T antigen synthesis was inhibited by more than 99.9%. This suggested that large T antigen was not required for the synthesis of late mRNA in mouse cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H. Transcription during productive infection with polyoma virus and Simian virus 40. Cell. 1976 May;8(1):1–12. doi: 10.1016/0092-8674(76)90179-3. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Khoury G. Effect of a tsA mutation of simian virus 40 late gene expression: variations between host cell lines. J Virol. 1980 Feb;33(2):920–925. doi: 10.1128/jvi.33.2.920-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwine J. C., Reed S. I., Stark G. R. Characterization of the autoregulation of simian virus 40 gene A. J Virol. 1977 Oct;24(1):22–27. doi: 10.1128/jvi.24.1.22-27.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier E. H., Chiu N., Radonovich M. F., May E., Salzman N. P. Regulation of simian virus 40 early and late gene transcription without viral DNA replication. J Virol. 1979 Mar;29(3):983–989. doi: 10.1128/jvi.29.3.983-989.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier E. H., May E., Salzman N. P. Characterization of simian virus 40 tsA58 transcriptional intermediates at restrictive temperatures: relationship between DNA replication and transcription. J Virol. 1977 Jun;22(3):702–710. doi: 10.1128/jvi.22.3.702-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Avila J., Martin R. G. Viral DNA synthesis in cells infected by temperature-sensitive mutants of simian virus 40. J Virol. 1974 Jul;14(1):116–124. doi: 10.1128/jvi.14.1.116-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cowan K., Tegtmeyer P., Anthony D. D. Relationship of replication and transcription of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1927–1930. doi: 10.1073/pnas.70.7.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Cole C. N., Smith A. E., Paucha E., Tegtmeyer P., Rundell K., Berg P. Organization and expression of early genes of simian virus 40. Proc Natl Acad Sci U S A. 1978 Jan;75(1):117–121. doi: 10.1073/pnas.75.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinand F. J., Brown M., Khoury G. Characterization of early simian virus 40 transcriptional complexes: late transcription in the absence of detectable DNA replication. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5443–5447. doi: 10.1073/pnas.74.12.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Swinscoe J., Lebowitz P., Weissman S. M. Heterogeneity and 5'-terminal structures of the late RNAs of simian virus 40. J Mol Biol. 1978 Dec 25;126(4):813–846. doi: 10.1016/0022-2836(78)90022-0. [DOI] [PubMed] [Google Scholar]
- Graessmann M., Graessmann A. Regulation mechanism of simian virus 40 late gene expression in primary kidney cells and simian virus 40 transformed 3T3 cells. Virology. 1975 Jun;65(2):591–594. doi: 10.1016/0042-6822(75)90066-5. [DOI] [PubMed] [Google Scholar]
- Haegeman G., Iserentant D., Gheysen D., Fiers W. Characterization of the major altered leader sequence of late mRNA induced by SV40 deletion mutant d1-1811. Nucleic Acids Res. 1979 Dec 11;7(7):1799–1814. doi: 10.1093/nar/7.7.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Sharp P. A. Expression of early and late simian virus 40 transcripts in the absence of protein synthesis. J Virol. 1980 Jun;34(3):592–597. doi: 10.1128/jvi.34.3.592-597.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. S. Gel electrophoretic separation of the complementary strands of bacteriophage DNA. Virology. 1972 Jul;49(1):342–344. doi: 10.1016/s0042-6822(72)80042-4. [DOI] [PubMed] [Google Scholar]
- Ito Y. Polyoma virus-specific 55K protein isolated from plasma membrane of productively infected cells is virus-coded and important for cell transformation. Virology. 1979 Oct 15;98(1):261–266. doi: 10.1016/0042-6822(79)90545-2. [DOI] [PubMed] [Google Scholar]
- Johnson L. F., Meister R. Rapid increase in poly(A) (+) mRNA content following inhibition of protein synthesis. J Cell Physiol. 1977 Jul;92(1):57–64. doi: 10.1002/jcp.1040920108. [DOI] [PubMed] [Google Scholar]
- Khoury G., Byrne J. C., Martin M. A. Patterns of Simian Virus 40 DNA transcription after acute infection of permissive and nonpermissive cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1925–1928. doi: 10.1073/pnas.69.7.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Howley P., Brown M., Martin M. The detection and quantitation of SV40 nucleic acid sequences using single-stranded SV40 DNA probes. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):147–152. doi: 10.1101/sqb.1974.039.01.020. [DOI] [PubMed] [Google Scholar]
- Khoury G., May E. Regulation of early and late simian virus 40 transcription: overproduction of early viral RNA in the absence of a functional T-antigen. J Virol. 1977 Jul;23(1):167–176. doi: 10.1128/jvi.23.1.167-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress M., May E., Cassingena R., May P. Simian virus 40-transformed cells express new species of proteins precipitable by anti-simian virus 40 tumor serum. J Virol. 1979 Aug;31(2):472–483. doi: 10.1128/jvi.31.2.472-483.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maroun L. E., Miller E. T. Increased messenger RNA from protein synthesis inhibited human fibroblasts. J Cell Physiol. 1977 Sep;92(3):375–379. doi: 10.1002/jcp.1040920306. [DOI] [PubMed] [Google Scholar]
- May E., Kress M., May P. Characterization of two SV40 early mRNAs and evidence for a nuclear "prespliced" RNA species. Nucleic Acids Res. 1978 Sep;5(9):3083–3099. doi: 10.1093/nar/5.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May E., May P., Weil R. "Early" virus-specific RNA may contain information necessary for chromosome replication and mitosis induced by Simian Virus 40. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1654–1658. doi: 10.1073/pnas.70.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P., Kress M., Lange M., May E. New genetic information expressed in SV40-transformed cells: characterization of the 55K proteins and evidence for unusual SV40 mRNAs. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):189–200. doi: 10.1101/sqb.1980.044.01.022. [DOI] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Piper P. W. Polyoma virus transcription early during productive infection of mouse 3T6 cells. J Mol Biol. 1979 Jun 25;131(2):399–407. doi: 10.1016/0022-2836(79)90083-4. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Ghosh P. K., Lebowitz P., Piatak M., Weissman S. M. Simian virus 40 early mRNA's. I. Genomic localization of 3' and 5' termini and two major splices in mRNA from transformed and lytically infected cells. J Virol. 1979 Apr;30(1):279–296. doi: 10.1128/jvi.30.1.279-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I., Stark G. R., Alwine J. C. Autoregulation of simian virus 40 gene A by T antigen. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3083–3087. doi: 10.1073/pnas.73.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio D., Robbins A., Myers R., Tjian R. Regulation of simian virus 40 early transcription in vitro by a purified tumor antigen. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5706–5710. doi: 10.1073/pnas.77.10.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal L. J. Isolation and characterization of poly(A)-containing polyoma "early" and "late" messenger RNAs. Nucleic Acids Res. 1976 Mar;3(3):661–676. doi: 10.1093/nar/3.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell K., Collins J. K., Tegtmeyer P., Ozer H. L., Lai C. J., Nathans D. Identification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):636–646. doi: 10.1128/jvi.21.2.636-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyzer M., Weil R., Frank G., Zuber H. Amino acid sequence analysis of fragments generated by partial proteolysis from large simian virus 40 tumor antigen. J Biol Chem. 1980 Jun 25;255(12):5627–5634. [PubMed] [Google Scholar]
- Tegtmeyer P. Altered patterns of protein synthesis in infection by SV40 mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):9–15. doi: 10.1101/sqb.1974.039.01.004. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Rundell K., Collins J. K. Modification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):647–657. doi: 10.1128/jvi.21.2.647-657.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia S. S. Immune response of rabbits to purified papovavirus SV40. J Immunol. 1970 Jan;104(1):72–78. [PubMed] [Google Scholar]
- Tournier P., Cassingena R., Wicker R., Coppey J., Suarez H. Etude du mécanisme de l'induction chez des cellules de hamster syrien transformées par le virus SV40. I. Propriétés d'une lignée cellulaire clonale. Int J Cancer. 1967 Mar 15;2(2):117–132. doi: 10.1002/ijc.2910020207. [DOI] [PubMed] [Google Scholar]
- Watkins J. F. The SV40 rescue problem. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):355–362. doi: 10.1101/sqb.1974.039.01.046. [DOI] [PubMed] [Google Scholar]
- Wilson M. C., Nevins J. R., Blanchard J. M., Ginsberg H. S., Darnell J. E., Jr Metabolism of mRNA from the transforming region of adenovirus 2. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):447–455. doi: 10.1101/sqb.1980.044.01.048. [DOI] [PubMed] [Google Scholar]