Abstract
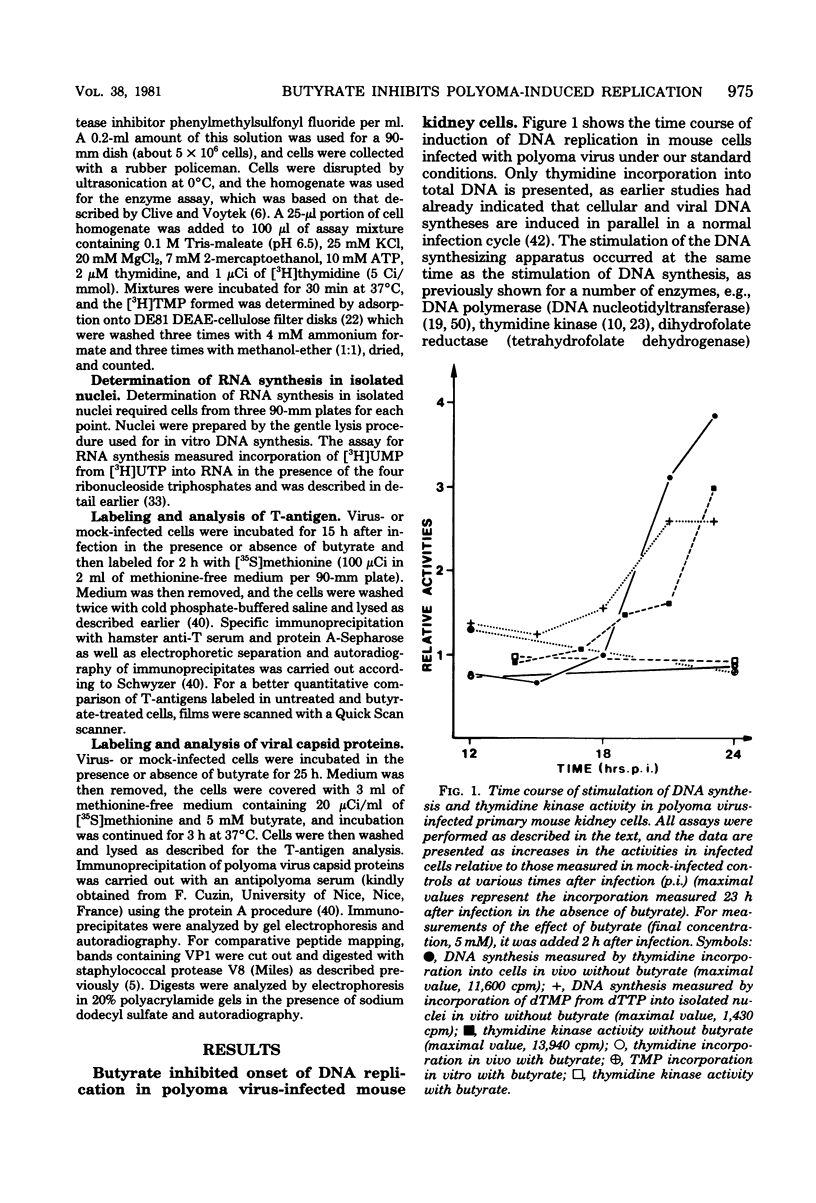
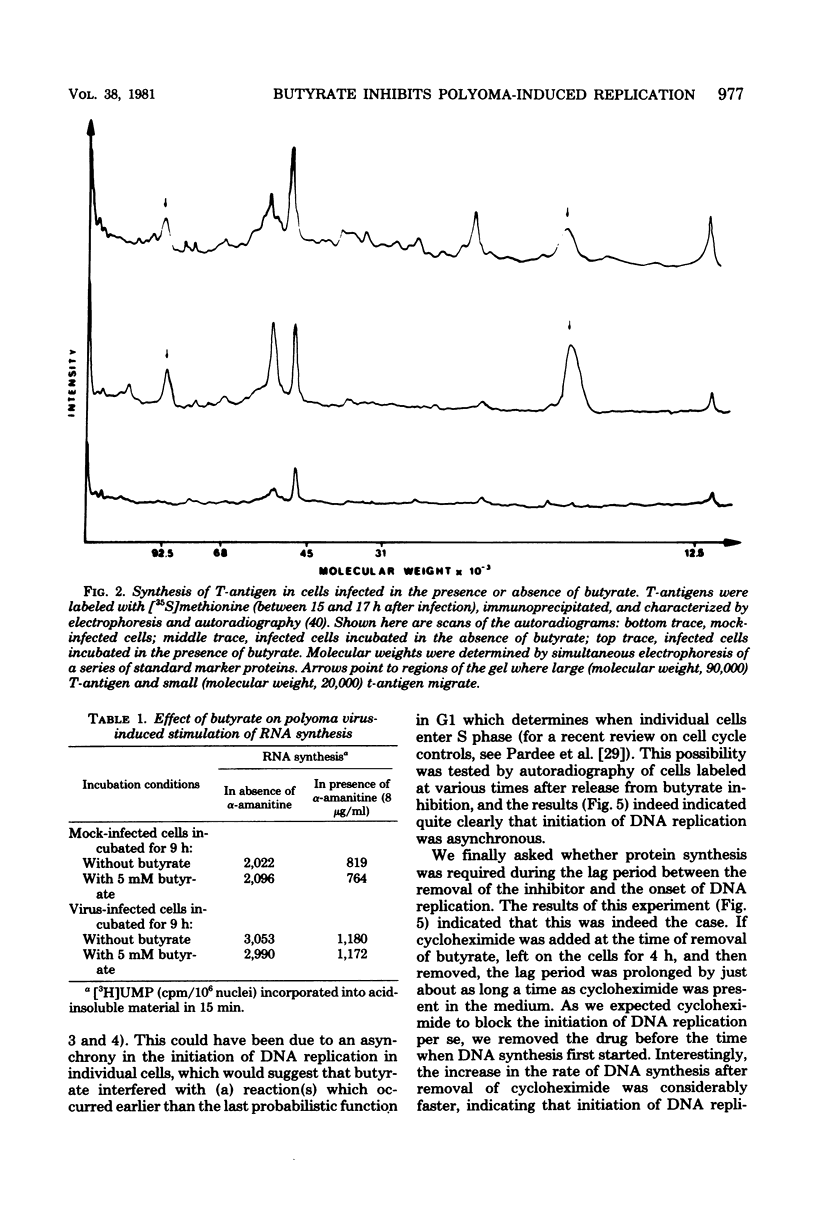
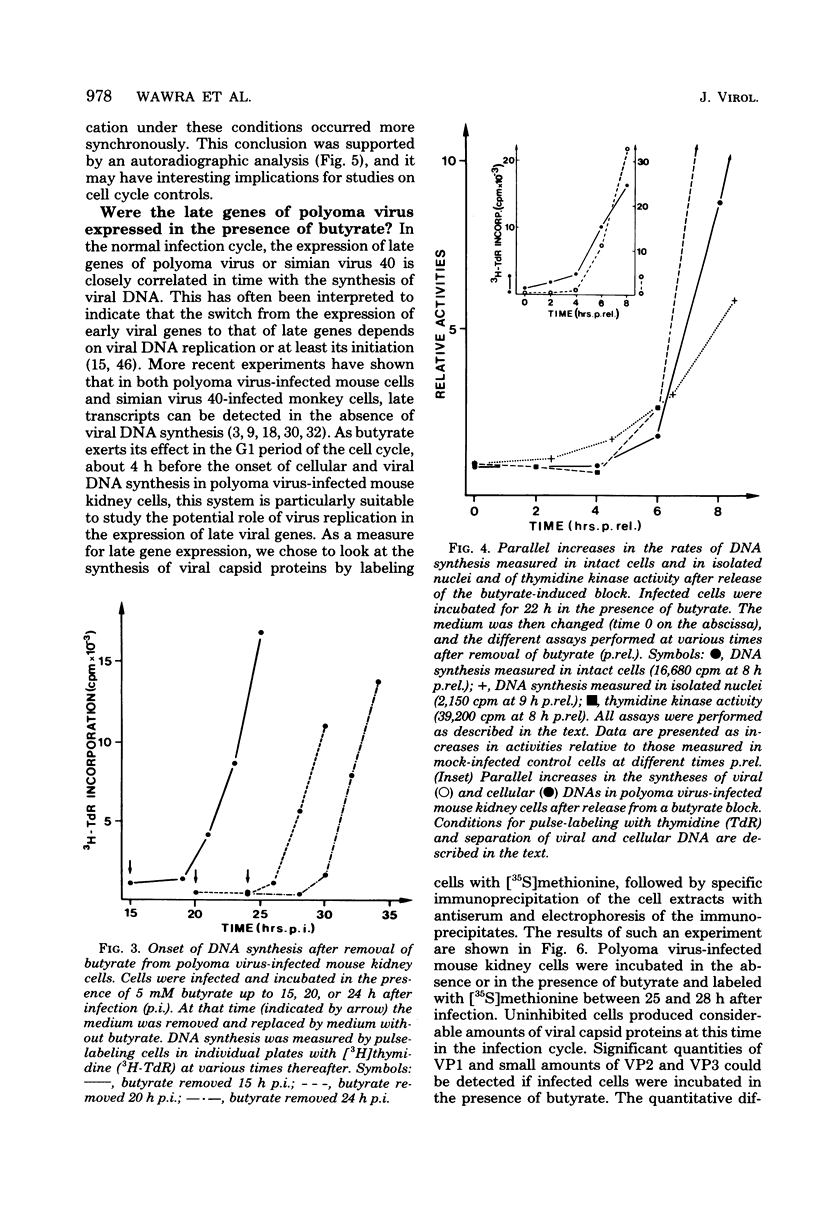
Sodium butyrate inhibited initiation of viral and cellular DNA replication in polyoma virus-infected mouse kidney cells. Ongoing viral or cellular DNA replication, however, was not affected by the presence of the substance. Butyrate had no effect on T-antigen synthesis and on the stimulation of transcription, one of the earliest reactions of the infected cells to the appearance of T-antigen, nor did it inhibit expression of late viral genes (synthesis of viral capsid proteins). In addition to blocking the onset of DNA synthesis, butyrate also inhibited stimulation of the activities of enzymes involved in DNA synthesis. When butyrate was removed, viral and cellular DNA syntheses were induced in parallel after a lag period of approximately 4 h. At the same time, the activities of enzymes involved in DNA synthesis increase. If protein synthesis was inhibited during part of the lag period, the initiation of DNA synthesis was retarded for the same time interval, suggesting that the proteins involved in the initiation of DNA replication had to be made. We have developed an in vitro system for measuring DNA synthesis in crude nuclear preparations which mimics the status of DNA replication in intact cells and may help in future experiments to study the requirements for initiation of cellular and viral DNA synthesis and the possible involvement of T-antigens in this reaction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin T. L. Host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1970 Sep;67(1):394–399. doi: 10.1073/pnas.67.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz W. C., Strominger J. L. Viral and cellular DNA synthesis in nuclei from human lymphocytes transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2413–2417. doi: 10.1073/pnas.72.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier E. H., Chiu N., Radonovich M. F., May E., Salzman N. P. Regulation of simian virus 40 early and late gene transcription without viral DNA replication. J Virol. 1979 Mar;29(3):983–989. doi: 10.1128/jvi.29.3.983-989.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido E. P., Reeves R., Davie J. R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Clive D., Voytek P. Evidence for chemically-induced structural gene mutations at the thymidine kinase locus in cultured L5178Y mouse lymphoma cells. Mutat Res. 1977 Aug;44(2):269–278. doi: 10.1016/0027-5107(77)90084-7. [DOI] [PubMed] [Google Scholar]
- FREARSON P. M., KIT S., DUBBS D. R. DEOXYTHYMIDYLATE SYNTHETASE AND DEOXYTHYMIDINE KINASE ACTIVITIES OF VIRUS-INFECTED ANIMAL CELLS. Cancer Res. 1965 Jun;25:737–744. [PubMed] [Google Scholar]
- FRIED M. ISOLATION OF TEMPERATURE-SENSITIVE MUTANTS OF POLYOMA VIRUS. Virology. 1965 Apr;25:669–671. doi: 10.1016/0042-6822(65)90098-x. [DOI] [PubMed] [Google Scholar]
- Fallon R. J., Cox R. P. Cell cycle analysis of sodium butyrate and hydroxyurea, inducers of ectopic hormone production in HeLa cells. J Cell Physiol. 1979 Aug;100(2):251–262. doi: 10.1002/jcp.1041000206. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Davoli D. Molecular biology of papovaviruses. Annu Rev Biochem. 1977;46:471–522. doi: 10.1146/annurev.bi.46.070177.002351. [DOI] [PubMed] [Google Scholar]
- Ferdinand F. J., Brown M., Khoury G. Characterization of early simian virus 40 transcriptional complexes: late transcription in the absence of detectable DNA replication. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5443–5447. doi: 10.1073/pnas.74.12.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frearson P. M., Kit S., Dubbs D. R. Induction of dihydrofolate reductase activity by SV40 and polyoma virus. Cancer Res. 1966 Aug;26(8):1653–1660. [PubMed] [Google Scholar]
- Gershey E. L. Simian virus 40-host cell interaction during lytic infection. J Virol. 1979 Apr;30(1):76–83. doi: 10.1128/jvi.30.1.76-83.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg E., Salomon D., Sreevalsan T., Freese E. Growth inhibition and morphological changes caused by lipophilic acids in mammalian cells. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2457–2461. doi: 10.1073/pnas.70.8.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Mueller C. Regulatory function of simian virus 40 DNA replication for late viral gene expression. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4831–4834. doi: 10.1073/pnas.74.11.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin B. E., Maddock C. New classes of viable deletion mutants in the early region of polyoma virus. J Virol. 1979 Sep;31(3):645–656. doi: 10.1128/jvi.31.3.645-656.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagopian H. K., Riggs M. G., Swartz L. A., Ingram V. M. Effect of n-butyrate on DNA synthesis in chick fibroblasts and HeLa cells. Cell. 1977 Nov;12(3):855–860. doi: 10.1016/0092-8674(77)90284-7. [DOI] [PubMed] [Google Scholar]
- Handa H., Sharp P. A. Expression of early and late simian virus 40 transcripts in the absence of protein synthesis. J Virol. 1980 Jun;34(3):592–597. doi: 10.1128/jvi.34.3.592-597.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Vogt M., Dulbecco R. Induction of cellular DNA synthesis by polyoma virus. II. Increase in the rate of enzyme synthesis after infection with polyoma virus in mouse kidney cells. Virology. 1965 Nov;27(3):262–272. doi: 10.1016/0042-6822(65)90105-4. [DOI] [PubMed] [Google Scholar]
- Henneberry R. C., Fishman P. H. Morphological and biochemical differentiation in HeLa cells. Effects of cycloheximide on butyrate-induced process formation and ganglioside metabolism. Exp Cell Res. 1976 Nov;103(1):55–62. doi: 10.1016/0014-4827(76)90240-8. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Subak-Sharpe J. H. Biochemical studies on the herpes simplex virus-specified deoxypyrimidine kinase activity. J Gen Virol. 1974 Sep;24(3):481–492. doi: 10.1099/0022-1317-24-3-481. [DOI] [PubMed] [Google Scholar]
- Khandjian E. W., Matter J. M., Léonard N., Weil R. Simian virus 40 and polyoma virus stimulate overall cellular RNA and protein synthesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1476–1480. doi: 10.1073/pnas.77.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kára J., Weil R. Specific activation of the DNA-synthesizing apparatus in contact-inhibited mouse kidney cells by polyoma virus. Proc Natl Acad Sci U S A. 1967 Jan;57(1):63–70. doi: 10.1073/pnas.57.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder A., Leder P. Butyric acid, a potent inducer of erythroid differentiation in cultured erythroleukemic cells. Cell. 1975 Jul;5(3):319–322. doi: 10.1016/0092-8674(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Lilley D. M., Berendt A. R. The gross level of in vitro RNA synthesis in HeLa nuclei is unaltered by histone hyperacetylation. Biochem Biophys Res Commun. 1979 Oct 12;90(3):917–924. doi: 10.1016/0006-291x(79)91915-6. [DOI] [PubMed] [Google Scholar]
- Loche M. P. Studies on polyoma virus DNA replication in synchronized C3H2K cells. J Gen Virol. 1979 Feb;42(2):429–433. doi: 10.1099/0022-1317-42-2-429. [DOI] [PubMed] [Google Scholar]
- Magnusson G., Berg P. Construction and analysis of viable deletion mutants of polyoma virus. J Virol. 1979 Nov;32(2):523–529. doi: 10.1128/jvi.32.2.523-529.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper P. W. Polyoma virus transcription early during productive infection of mouse 3T6 cells. J Mol Biol. 1979 Jun 25;131(2):399–407. doi: 10.1016/0022-2836(79)90083-4. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Sinha P. K. Effect of sodium butyrate on mammalian cells in culture: a review. In Vitro. 1976 Feb;12(2):125–132. doi: 10.1007/BF02796360. [DOI] [PubMed] [Google Scholar]
- Pétursson G., Weil R. A study on the mechanism of polyoma-induced activation of the cellular DNA-synthesizing apparatus. Synchronization by FUdR of virus-induced DNA synthesis. Arch Gesamte Virusforsch. 1968;24(1):1–29. doi: 10.1007/BF01242898. [DOI] [PubMed] [Google Scholar]
- Pöckl E., Wintersberger E. Increased rate of RNA synthesis: early reaction of primary mouse kidney cells to infection with polyoma virus of simian virus 40. J Virol. 1980 Jul;35(1):8–19. doi: 10.1128/jvi.35.1.8-19.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Rubenstein P., Sealy L., Marshall S., Chalkley R. Cellular protein synthesis and inhibition of cell division are independent of butyrate-induced histone hyperacetylation. Nature. 1979 Aug 23;280(5724):692–693. doi: 10.1038/280692a0. [DOI] [PubMed] [Google Scholar]
- Salomon C., Türler H., Weil R. Polyoma-induced stimulation of cellular RNA synthesis is paralleled by changed expression of the viral genome. Nucleic Acids Res. 1977;4(5):1483–1503. doi: 10.1093/nar/4.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Shatkin A. J. Polynucleotide ligase activity in cells infected with simian virus 40, polyoma virus, or vaccinia virus. J Virol. 1969 Nov;4(5):719–726. doi: 10.1128/jvi.4.5.719-726.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F. H. Effects of sodium butyrate on mouse neuroblastoma cells in culture. Biochem Pharmacol. 1976 Oct 15;25(20):2309–2317. doi: 10.1016/0006-2952(76)90015-0. [DOI] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978 May;14(1):115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- Seebeck T., Weil R. Polyoma viral DNA replicated as a nucleoprotein complex in close association with the host cell chromatin. J Virol. 1974 Mar;13(3):567–576. doi: 10.1128/jvi.13.3.567-576.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil R., Salomon E., May E., May P. A simplifying concept in tumor virology: virus-specific "pleiotropic effectors". Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):381–395. doi: 10.1101/sqb.1974.039.01.050. [DOI] [PubMed] [Google Scholar]
- Weil R. Viral 'tumor antigens': A novel type of mammalian regulator protein. Biochim Biophys Acta. 1978 Nov 17;516(3):301–388. doi: 10.1016/0304-419x(78)90012-4. [DOI] [PubMed] [Google Scholar]
- Wintersberger U., Wintersberger E. DNA polymerases in polyoma virus-infected mouse kidney cells. J Virol. 1975 Nov;16(5):1095–1100. doi: 10.1128/jvi.16.5.1095-1100.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouzias D., Basilico C. T-antigen expression in proliferating and non-proliferating simian virus 40-transformed mouse cells. J Virol. 1979 Jun;30(3):711–719. doi: 10.1128/jvi.30.3.711-719.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]