Abstract
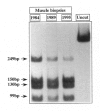
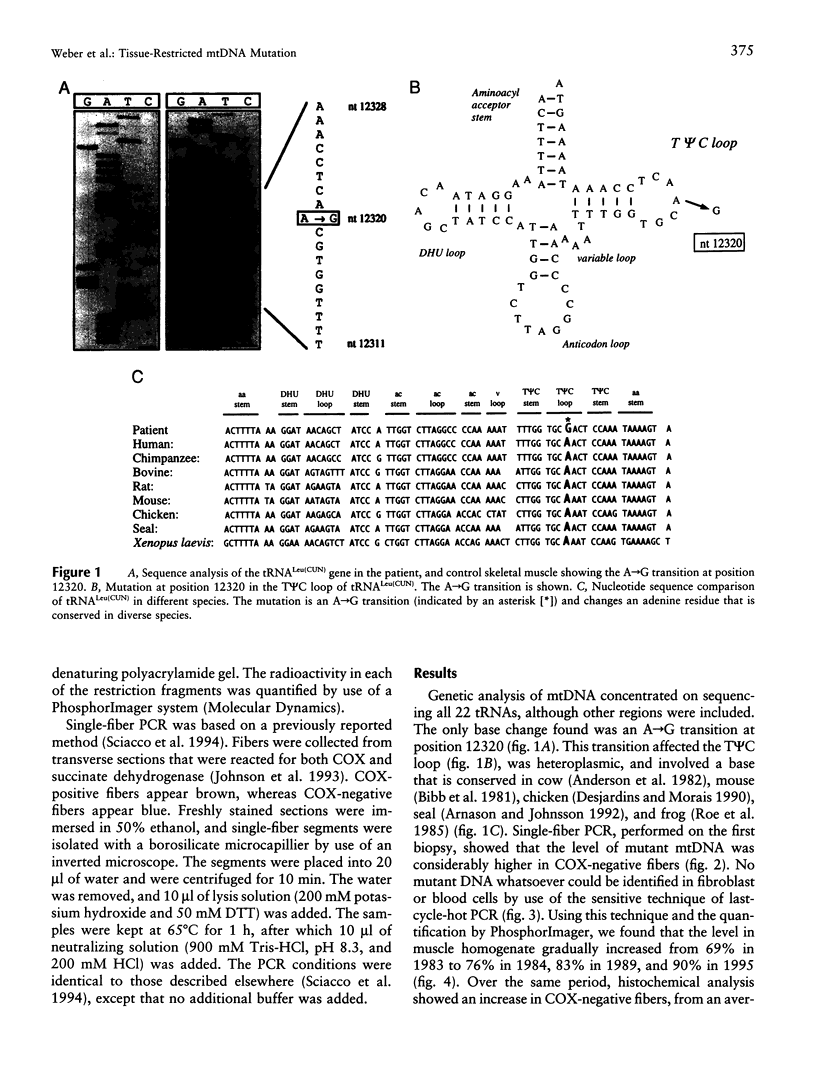
We have identified a new mutation in mtDNA, involving tRNALeu(CUN) in a patient manifesting an isolated skeletal myopathy. This heteroplasmic A-->G transition at position 12320 affects the T psi C loop at a conserved site and was not found in 120 controls. Analysis of cultured fibroblasts, white blood cells/platelets, and skeletal muscle showed that only skeletal muscle contained the mutation and that only this tissue demonstrated a biochemical defect of respiratory-chain activity. In a series of four muscle-biopsy specimens taken over a 12-year period, there was a gradual increase, from 70% to 90%, in the overall level of mutation, as well as a marked clinical deterioration. Single-fiber PCR confirmed that the proportion of mutant mtDNA was highest in cytochrome c oxidase-negative fibers. This study, which reports a mutation involving tRNALeu(CUN), demonstrates clearly that mtDNA point mutations can accumulate over time and may be restricted in their tissue distribution. Furthermore, clinical deterioration seemed to follow the increase in the level of mutation, although, interestingly, the appearance of fibers deficient in respiratory-chain activity showed a lag period.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Arnason U., Johnsson E. The complete mitochondrial DNA sequence of the harbor seal, Phoca vitulina. J Mol Evol. 1992 Jun;34(6):493–505. doi: 10.1007/BF00160463. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins P., Morais R. Sequence and gene organization of the chicken mitochondrial genome. A novel gene order in higher vertebrates. J Mol Biol. 1990 Apr 20;212(4):599–634. doi: 10.1016/0022-2836(90)90225-B. [DOI] [PubMed] [Google Scholar]
- Dunbar D. R., Moonie P. A., Jacobs H. T., Holt I. J. Different cellular backgrounds confer a marked advantage to either mutant or wild-type mitochondrial genomes. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6562–6566. doi: 10.1073/pnas.92.14.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Hayashi J., Ohta S., Kagawa Y., Takai D., Miyabayashi S., Tada K., Fukushima H., Inui K., Okada S., Goto Y. Functional and morphological abnormalities of mitochondria in human cells containing mitochondrial DNA with pathogenic point mutations in tRNA genes. J Biol Chem. 1994 Jul 22;269(29):19060–19066. [PubMed] [Google Scholar]
- Howell N., Bindoff L. A., McCullough D. A., Kubacka I., Poulton J., Mackey D., Taylor L., Turnbull D. M. Leber hereditary optic neuropathy: identification of the same mitochondrial ND1 mutation in six pedigrees. Am J Hum Genet. 1991 Nov;49(5):939–950. [PMC free article] [PubMed] [Google Scholar]
- Jackson M. J., Bindoff L. A., Weber K., Wilson J. N., Ince P., Alberti K. G., Turnbull D. M. Biochemical and molecular studies of mitochondrial function in diabetes insipidus, diabetes mellitus, optic atrophy, and deafness. Diabetes Care. 1994 Jul;17(7):728–733. doi: 10.2337/diacare.17.7.728. [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Bindoff L. A., Turnbull D. M. Cytochrome c oxidase activity in single muscle fibers: assay techniques and diagnostic applications. Ann Neurol. 1993 Jan;33(1):28–35. doi: 10.1002/ana.410330106. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Sakuta R., Hashimoto K., Fujino O., Fujita T., Hida M., Horai S., Goto Y., Nonaka I. Mitochondrial myopathy with progressive decrease in mitochondrial tRNA(Leu)(UUR) mutant genomes. Ann Neurol. 1994 Mar;35(3):370–373. doi: 10.1002/ana.410350322. [DOI] [PubMed] [Google Scholar]
- King M. P., Koga Y., Davidson M., Schon E. A. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNA(Leu(UUR)) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Mol Cell Biol. 1992 Feb;12(2):480–490. doi: 10.1128/mcb.12.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson N. G., Holme E., Kristiansson B., Oldfors A., Tulinius M. Progressive increase of the mutated mitochondrial DNA fraction in Kearns-Sayre syndrome. Pediatr Res. 1990 Aug;28(2):131–136. doi: 10.1203/00006450-199008000-00011. [DOI] [PubMed] [Google Scholar]
- Lowerson S. A., Taylor L., Briggs H. L., Turnbull D. M. Measurement of the activity of individual respiratory chain complexes in isolated fibroblast mitochondria. Anal Biochem. 1992 Sep;205(2):372–374. doi: 10.1016/0003-2697(92)90453-e. [DOI] [PubMed] [Google Scholar]
- Mariotti C., Tiranti V., Carrara F., Dallapiccola B., DiDonato S., Zeviani M. Defective respiratory capacity and mitochondrial protein synthesis in transformant cybrids harboring the tRNA(Leu(UUR)) mutation associated with maternally inherited myopathy and cardiomyopathy. J Clin Invest. 1994 Mar;93(3):1102–1107. doi: 10.1172/JCI117061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merante F., Tein I., Benson L., Robinson B. H. Maternally inherited hypertrophic cardiomyopathy due to a novel T-to-C transition at nucleotide 9997 in the mitochondrial tRNA(glycine) gene. Am J Hum Genet. 1994 Sep;55(3):437–446. [PMC free article] [PubMed] [Google Scholar]
- Moraes C. T., Ciacci F., Bonilla E., Ionasescu V., Schon E. A., DiMauro S. A mitochondrial tRNA anticodon swap associated with a muscle disease. Nat Genet. 1993 Jul;4(3):284–288. doi: 10.1038/ng0793-284. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., Ciacci F., Bonilla E., Jansen C., Hirano M., Rao N., Lovelace R. E., Rowland L. P., Schon E. A., DiMauro S. Two novel pathogenic mitochondrial DNA mutations affecting organelle number and protein synthesis. Is the tRNA(Leu(UUR)) gene an etiologic hot spot? J Clin Invest. 1993 Dec;92(6):2906–2915. doi: 10.1172/JCI116913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton J., Deadman M. E., Bindoff L., Morten K., Land J., Brown G. Families of mtDNA re-arrangements can be detected in patients with mtDNA deletions: duplications may be a transient intermediate form. Hum Mol Genet. 1993 Jan;2(1):23–30. doi: 10.1093/hmg/2.1.23. [DOI] [PubMed] [Google Scholar]
- Reid F. M., Vernham G. A., Jacobs H. T. A novel mitochondrial point mutation in a maternal pedigree with sensorineural deafness. Hum Mutat. 1994;3(3):243–247. doi: 10.1002/humu.1380030311. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Sciacco M., Bonilla E., Schon E. A., DiMauro S., Moraes C. T. Distribution of wild-type and common deletion forms of mtDNA in normal and respiration-deficient muscle fibers from patients with mitochondrial myopathy. Hum Mol Genet. 1994 Jan;3(1):13–19. doi: 10.1093/hmg/3.1.13. [DOI] [PubMed] [Google Scholar]
- Seibel P., Lauber J., Klopstock T., Marsac C., Kadenbach B., Reichmann H. Chronic progressive external ophthalmoplegia is associated with a novel mutation in the mitochondrial tRNA(Asn) gene. Biochem Biophys Res Commun. 1994 Oct 28;204(2):482–489. doi: 10.1006/bbrc.1994.2485. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Ino H., Ohno K., Hattori K., Sato W., Ozawa T., Tanaka T., Itoyama S. Mitochondrial mutation in fatal infantile cardiomyopathy. Lancet. 1990 Dec 8;336(8728):1452–1452. doi: 10.1016/0140-6736(90)93162-i. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Assignment of the chloramphenicol resistance gene to mitochondrial deoxyribonucleic acid and analysis of its expression in cultured human cells. Mol Cell Biol. 1981 Aug;1(8):697–710. doi: 10.1128/mcb.1.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda M., Chomyn A., Martinuzzi A., Hurko O., Attardi G. Marked replicative advantage of human mtDNA carrying a point mutation that causes the MELAS encephalomyopathy. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11164–11168. doi: 10.1073/pnas.89.23.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. L., Aprille J. R., Ernst S. G. Mitochondrial tRNA(thr) mutation in fatal infantile respiratory enzyme deficiency. Biochem Biophys Res Commun. 1991 May 15;176(3):1112–1115. doi: 10.1016/0006-291x(91)90399-r. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Amati P., Bresolin N., Antozzi C., Piccolo G., Toscano A., DiDonato S. Rapid detection of the A----G(8344) mutation of mtDNA in Italian families with myoclonus epilepsy and ragged-red fibers (MERRF). Am J Hum Genet. 1991 Feb;48(2):203–211. [PMC free article] [PubMed] [Google Scholar]