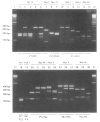
Abstract
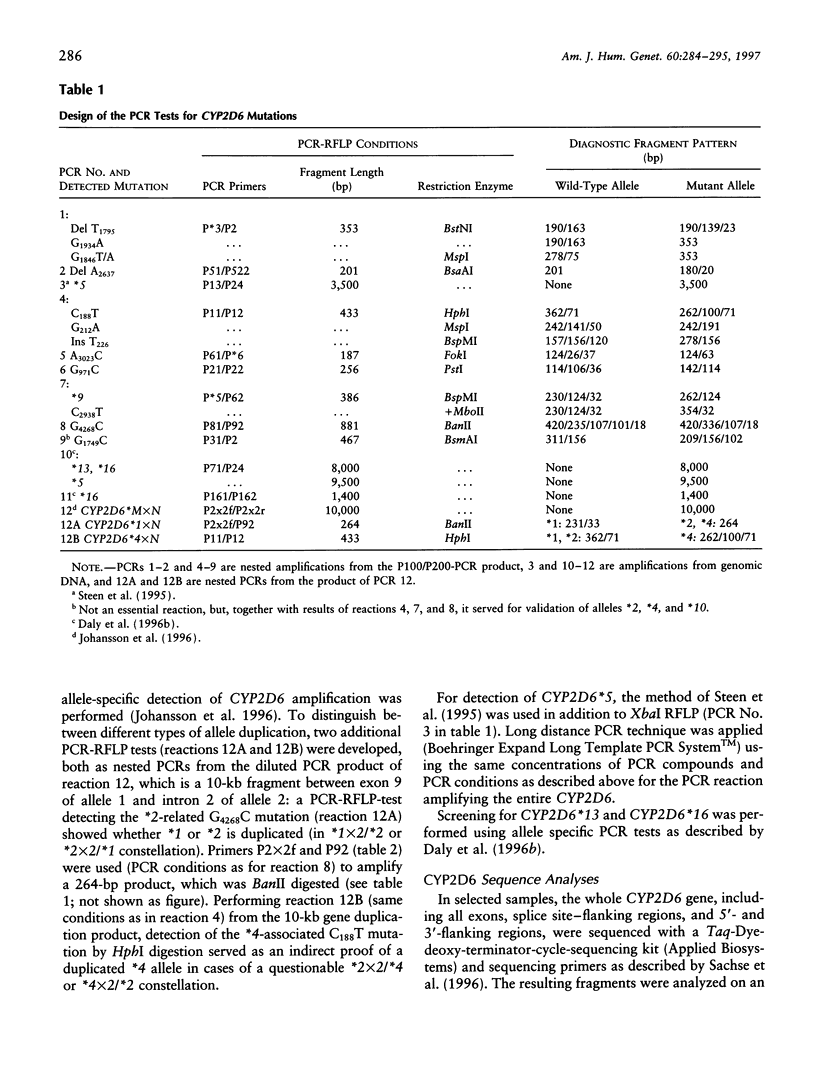
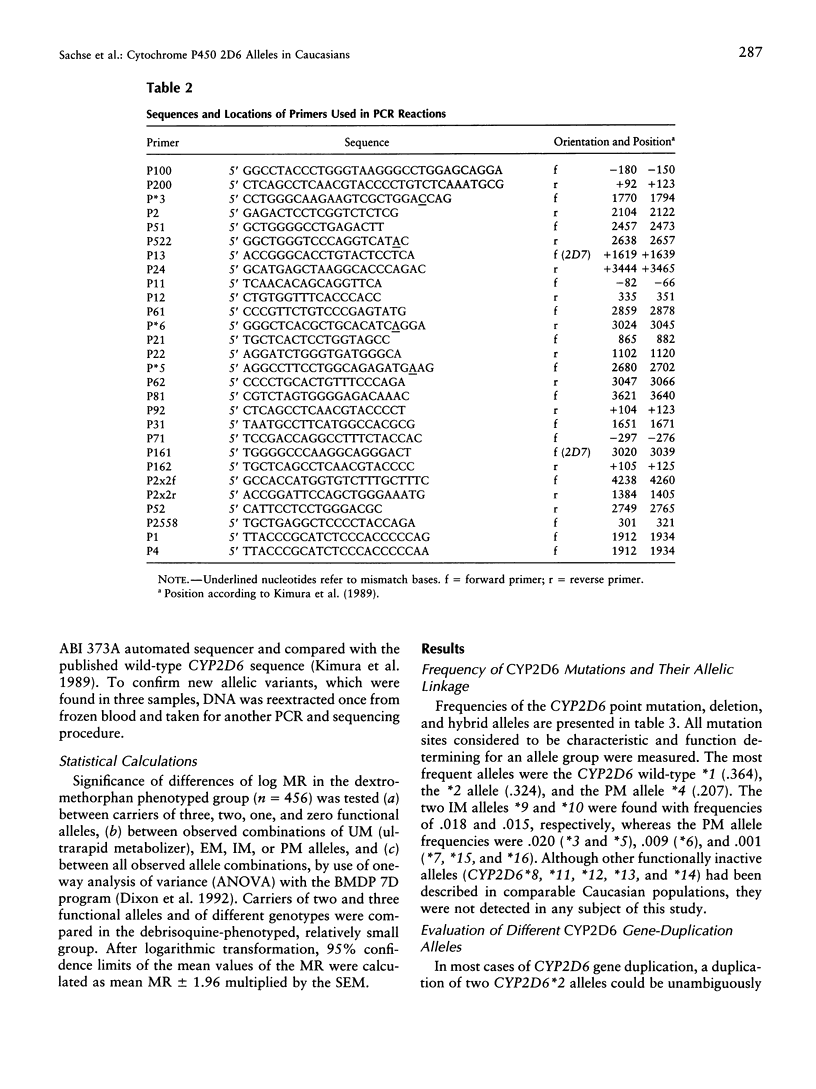
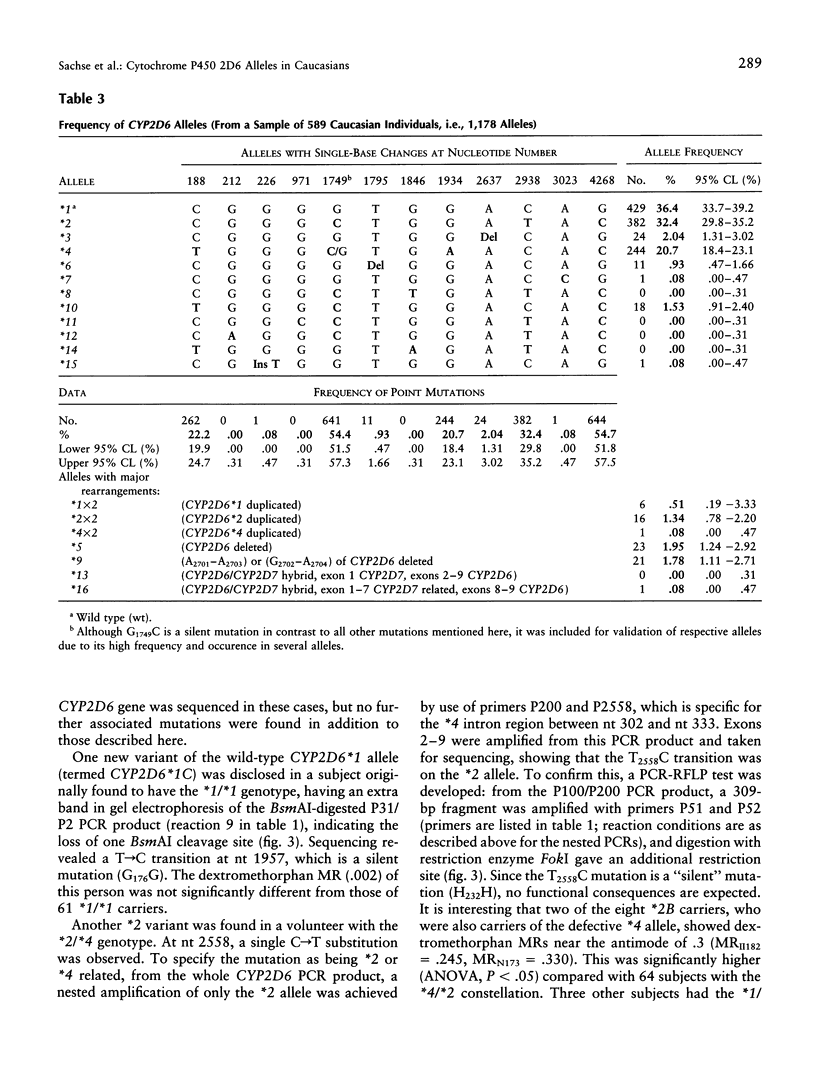
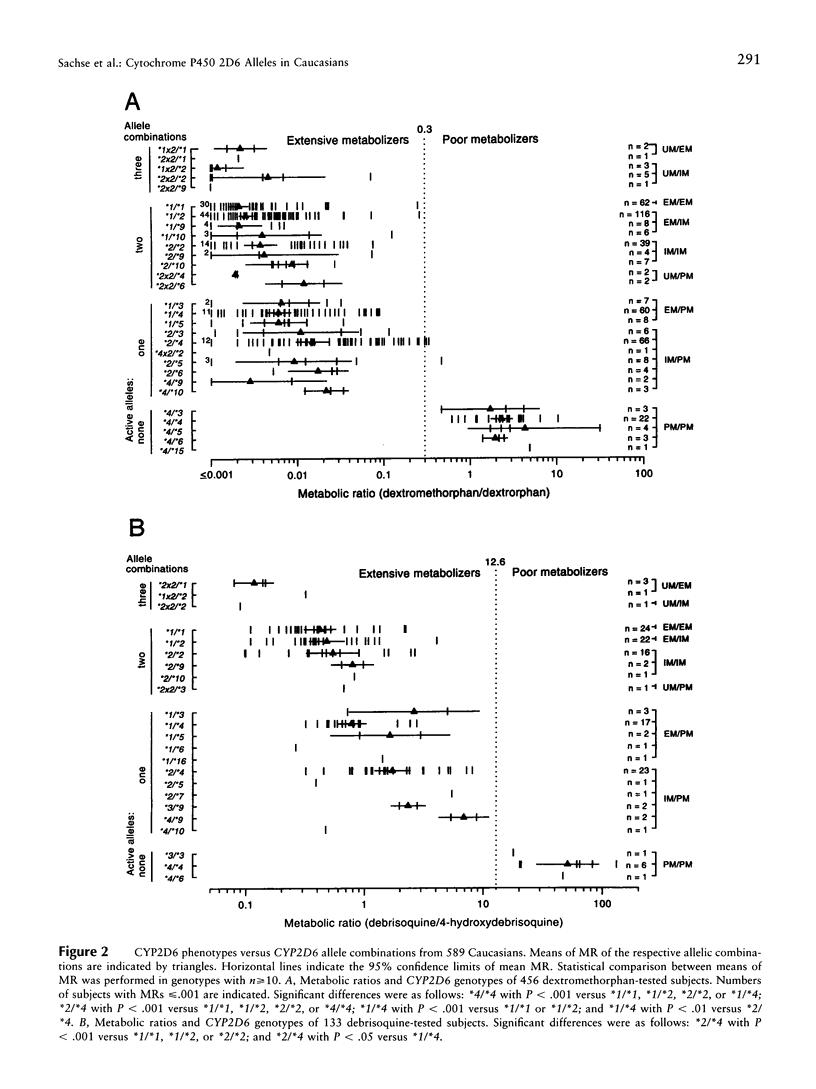
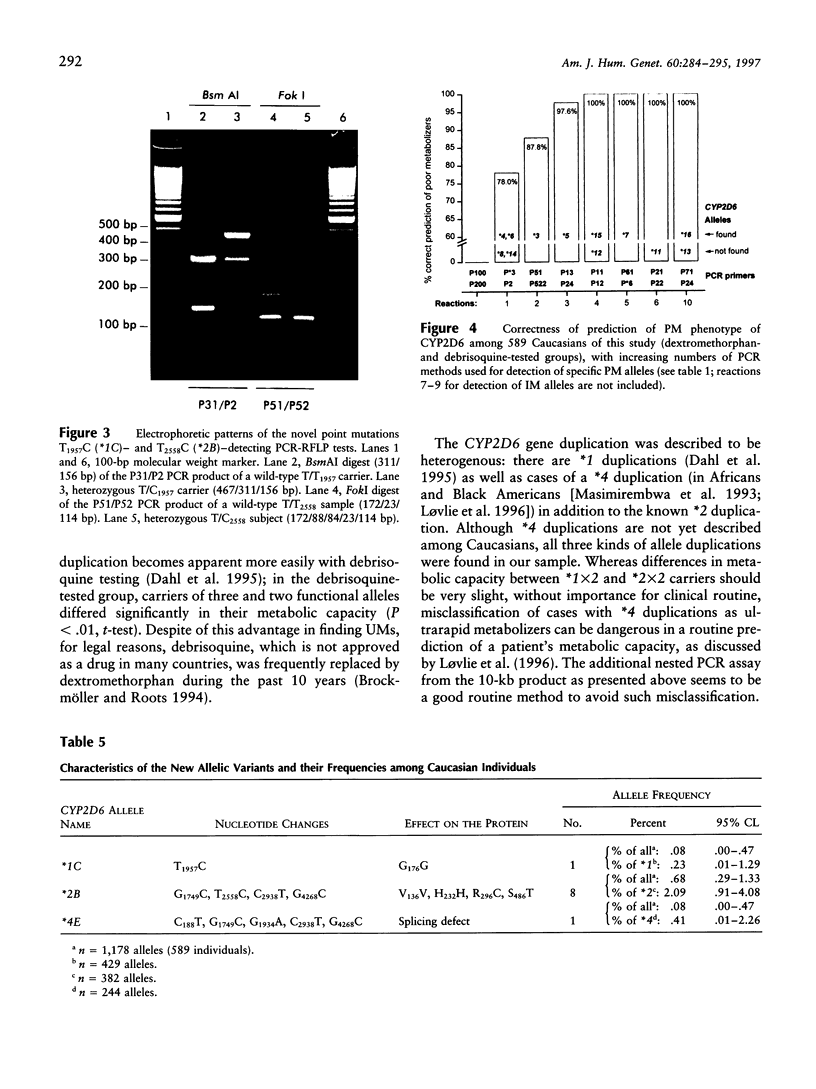
Cytochrome P450 2D6 (CYP2D6) metabolizes many important drugs. CYP2D6 activity ranges from complete deficiency to ultrafast metabolism, depending on at least 16 different known alleles. Their frequencies were determined in 589 unrelated German volunteers and correlated with enzyme activity measured by phenotyping with dextromethorphan or debrisoquine. For genotyping, nested PCR-RFLP tests from a PCR amplificate of the entire CYP2D6 gene were developed. The frequency of the CYP2D6*1 allele coding for extensive metabolizer (EM) phenotype was .364. The alleles coding for slightly (CYP2D6*2) or moderately (*9 and *10) reduced activity (intermediate metabolizer phenotype [IM]) showed frequencies of .324, .018, and .015, respectively. By use of novel PCR tests for discrimination, CYP2D6 gene duplication alleles were found with frequencies of .005 (*1x2), .013 (*2x2), and .001 (*4x2). Frequencies of alleles with complete deficiency (poor metabolizer phenotype [PM]) were .207 (*4), .020 (*3 and *5), .009 (*6), and .001 (*7, *15, and *16). The defective CYP2D6 alleles *8, *11, *12, *13, and *14 were not found. All 41 PMs (7.0%) in this sample were explained by five mutations detected by four PCR-RFLP tests, which may suffice, together with the gene duplication test, for clinical prediction of CYP2D6 capacity. Three novel variants of known CYP2D6 alleles were discovered: *1C (T1957C), *2B (additional C2558T), and *4E (additional C2938T). Analysis of variance showed significant differences in enzymatic activity measured by the dextromethorphan metabolic ratio (MR) between carriers of EM/PM (mean MR = .006) and IM/PM (mean MR = .014) alleles and between carriers of one (mean MR = .009) and two (mean MR = .003) functional alleles. The results of this study provide a solid basis for prediction of CYP2D6 capacity, as required in drug research and routine drug treatment.
Full text
PDF





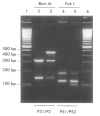
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agúndez J. A., Ledesma M. C., Ladero J. M., Benítez J. Prevalence of CYP2D6 gene duplication and its repercussion on the oxidative phenotype in a white population. Clin Pharmacol Ther. 1995 Mar;57(3):265–269. doi: 10.1016/0009-9236(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Aklillu E., Persson I., Bertilsson L., Johansson I., Rodrigues F., Ingelman-Sundberg M. Frequent distribution of ultrarapid metabolizers of debrisoquine in an ethiopian population carrying duplicated and multiduplicated functional CYP2D6 alleles. J Pharmacol Exp Ther. 1996 Jul;278(1):441–446. [PubMed] [Google Scholar]
- Appanna G., Tang B. K., Mller R., Kalow W. A sensitive method for determination of cytochrome P4502D6 activity in vitro using bupranolol as substrate. Drug Metab Dispos. 1996 Mar;24(3):303–306. [PubMed] [Google Scholar]
- Bertilsson L. Geographical/interracial differences in polymorphic drug oxidation. Current state of knowledge of cytochromes P450 (CYP) 2D6 and 2C19. Clin Pharmacokinet. 1995 Sep;29(3):192–209. doi: 10.2165/00003088-199529030-00005. [DOI] [PubMed] [Google Scholar]
- Brockmöller J., Roots I. Assessment of liver metabolic function. Clinical implications. Clin Pharmacokinet. 1994 Sep;27(3):216–248. doi: 10.2165/00003088-199427030-00005. [DOI] [PubMed] [Google Scholar]
- Broly F., Gaedigk A., Heim M., Eichelbaum M., Morike K., Meyer U. A. Debrisoquine/sparteine hydroxylation genotype and phenotype: analysis of common mutations and alleles of CYP2D6 in a European population. DNA Cell Biol. 1991 Oct;10(8):545–558. doi: 10.1089/dna.1991.10.545. [DOI] [PubMed] [Google Scholar]
- Chen Z. R., Somogyi A. A., Bochner F. Simultaneous determination of dextromethorphan and three metabolites in plasma and urine using high-performance liquid chromatography with application to their disposition in man. Ther Drug Monit. 1990 Jan;12(1):97–104. doi: 10.1097/00007691-199001000-00018. [DOI] [PubMed] [Google Scholar]
- Dahl M. L., Johansson I., Bertilsson L., Ingelman-Sundberg M., Sjöqvist F. Ultrarapid hydroxylation of debrisoquine in a Swedish population. Analysis of the molecular genetic basis. J Pharmacol Exp Ther. 1995 Jul;274(1):516–520. [PubMed] [Google Scholar]
- Daly A. K., Armstrong M., Monkman S. C., Idle M. E., Idle J. R. Genetic and metabolic criteria for the assignment of debrisoquine 4-hydroxylation (cytochrome P4502D6) phenotypes. Pharmacogenetics. 1991 Oct;1(1):33–41. doi: 10.1097/00008571-199110000-00006. [DOI] [PubMed] [Google Scholar]
- Daly A. K., Brockmöller J., Broly F., Eichelbaum M., Evans W. E., Gonzalez F. J., Huang J. D., Idle J. R., Ingelman-Sundberg M., Ishizaki T. Nomenclature for human CYP2D6 alleles. Pharmacogenetics. 1996 Jun;6(3):193–201. doi: 10.1097/00008571-199606000-00001. [DOI] [PubMed] [Google Scholar]
- Daly A. K., Fairbrother K. S., Andreassen O. A., London S. J., Idle J. R., Steen V. M. Characterization and PCR-based detection of two different hybrid CYP2D7P/CYP2D6 alleles associated with the poor metabolizer phenotype. Pharmacogenetics. 1996 Aug;6(4):319–328. doi: 10.1097/00008571-199608000-00005. [DOI] [PubMed] [Google Scholar]
- Daly A. K., Leathart J. B., London S. J., Idle J. R. An inactive cytochrome P450 CYP2D6 allele containing a deletion and a base substitution. Hum Genet. 1995 Mar;95(3):337–341. doi: 10.1007/BF00225204. [DOI] [PubMed] [Google Scholar]
- Evert B., Griese E. U., Eichelbaum M. Cloning and sequencing of a new non-functional CYP2D6 allele: deletion of T1795 in exon 3 generates a premature stop codon. Pharmacogenetics. 1994 Oct;4(5):271–274. doi: 10.1097/00008571-199410000-00005. [DOI] [PubMed] [Google Scholar]
- Gough A. C., Miles J. S., Spurr N. K., Moss J. E., Gaedigk A., Eichelbaum M., Wolf C. R. Identification of the primary gene defect at the cytochrome P450 CYP2D locus. Nature. 1990 Oct 25;347(6295):773–776. doi: 10.1038/347773a0. [DOI] [PubMed] [Google Scholar]
- Heim M., Meyer U. A. Genotyping of poor metabolisers of debrisoquine by allele-specific PCR amplification. Lancet. 1990 Sep 1;336(8714):529–532. doi: 10.1016/0140-6736(90)92086-w. [DOI] [PubMed] [Google Scholar]
- Johansson I., Lundqvist E., Bertilsson L., Dahl M. L., Sjöqvist F., Ingelman-Sundberg M. Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultrarapid metabolism of debrisoquine. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11825–11829. doi: 10.1073/pnas.90.24.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I., Lundqvist E., Dahl M. L., Ingelman-Sundberg M. PCR-based genotyping for duplicated and deleted CYP2D6 genes. Pharmacogenetics. 1996 Aug;6(4):351–355. doi: 10.1097/00008571-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Kimura S., Umeno M., Skoda R. C., Meyer U. A., Gonzalez F. J. The human debrisoquine 4-hydroxylase (CYP2D) locus: sequence and identification of the polymorphic CYP2D6 gene, a related gene, and a pseudogene. Am J Hum Genet. 1989 Dec;45(6):889–904. [PMC free article] [PubMed] [Google Scholar]
- Lennard M. S., Silas J. H., Smith A. J., Tucker G. T. Determination of debrisoquine and its 4-hydroxy metabolite in biological fluids by gas chromatography with flame-ionization and nitrogen-selective detection. J Chromatogr. 1977 Mar 11;133(1):161–166. doi: 10.1016/s0021-9673(00)89216-x. [DOI] [PubMed] [Google Scholar]
- Løvlie R., Daly A. K., Molven A., Idle J. R., Steen V. M. Ultrarapid metabolizers of debrisoquine: characterization and PCR-based detection of alleles with duplication of the CYP2D6 gene. FEBS Lett. 1996 Aug 19;392(1):30–34. doi: 10.1016/0014-5793(96)00779-x. [DOI] [PubMed] [Google Scholar]
- Mahgoub A., Idle J. R., Dring L. G., Lancaster R., Smith R. L. Polymorphic hydroxylation of Debrisoquine in man. Lancet. 1977 Sep 17;2(8038):584–586. doi: 10.1016/s0140-6736(77)91430-1. [DOI] [PubMed] [Google Scholar]
- Masimirembwa C. M., Johansson I., Hasler J. A., Ingelman-Sundberg M. Genetic polymorphism of cytochrome P450 CYP2D6 in Zimbabwean population. Pharmacogenetics. 1993 Dec;3(6):275–280. doi: 10.1097/00008571-199312000-00001. [DOI] [PubMed] [Google Scholar]
- Panserat S., Mura C., Gérard N., Vincent-Viry M., Galteau M. M., Jacoz-Aigrain E., Krishnamoorthy R. An unequal cross-over event within the CYP2D gene cluster generates a chimeric CYP2D7/CYP2D6 gene which is associated with the poor metabolizer phenotype. Br J Clin Pharmacol. 1995 Oct;40(4):361–367. doi: 10.1111/j.1365-2125.1995.tb04558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse C., Brockmöller J., Bauer S., Reum T., Roots I. A rare insertion of T226 in exon 1 of CYP2D6 causes a frameshift and is associated with the poor metabolizer phenotype: CYP2D6*15. Pharmacogenetics. 1996 Jun;6(3):269–272. doi: 10.1097/00008571-199606000-00012. [DOI] [PubMed] [Google Scholar]
- Saxena R., Shaw G. L., Relling M. V., Frame J. N., Moir D. T., Evans W. E., Caporaso N., Weiffenbach B. Identification of a new variant CYP2D6 allele with a single base deletion in exon 3 and its association with the poor metabolizer phenotype. Hum Mol Genet. 1994 Jun;3(6):923–926. doi: 10.1093/hmg/3.6.923. [DOI] [PubMed] [Google Scholar]
- Schmid B., Bircher J., Preisig R., Küpfer A. Polymorphic dextromethorphan metabolism: co-segregation of oxidative O-demethylation with debrisoquin hydroxylation. Clin Pharmacol Ther. 1985 Dec;38(6):618–624. doi: 10.1038/clpt.1985.235. [DOI] [PubMed] [Google Scholar]
- Skoda R. C., Gonzalez F. J., Demierre A., Meyer U. A. Two mutant alleles of the human cytochrome P-450db1 gene (P450C2D1) associated with genetically deficient metabolism of debrisoquine and other drugs. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5240–5243. doi: 10.1073/pnas.85.14.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen V. M., Andreassen O. A., Daly A. K., Tefre T., Børresen A. L., Idle J. R., Gulbrandsen A. K. Detection of the poor metabolizer-associated CYP2D6(D) gene deletion allele by long-PCR technology. Pharmacogenetics. 1995 Aug;5(4):215–223. doi: 10.1097/00008571-199508000-00005. [DOI] [PubMed] [Google Scholar]
- Tefre T., Daly A. K., Armstrong M., Leathart J. B., Idle J. R., Brøgger A., Børresen A. L. Genotyping of the CYP2D6 gene in Norwegian lung cancer patients and controls. Pharmacogenetics. 1994 Apr;4(2):47–57. [PubMed] [Google Scholar]
- Tyndale R., Aoyama T., Broly F., Matsunaga T., Inaba T., Kalow W., Gelboin H. V., Meyer U. A., Gonzalez F. J. Identification of a new variant CYP2D6 allele lacking the codon encoding Lys-281: possible association with the poor metabolizer phenotype. Pharmacogenetics. 1991 Oct;1(1):26–32. doi: 10.1097/00008571-199110000-00005. [DOI] [PubMed] [Google Scholar]