Abstract
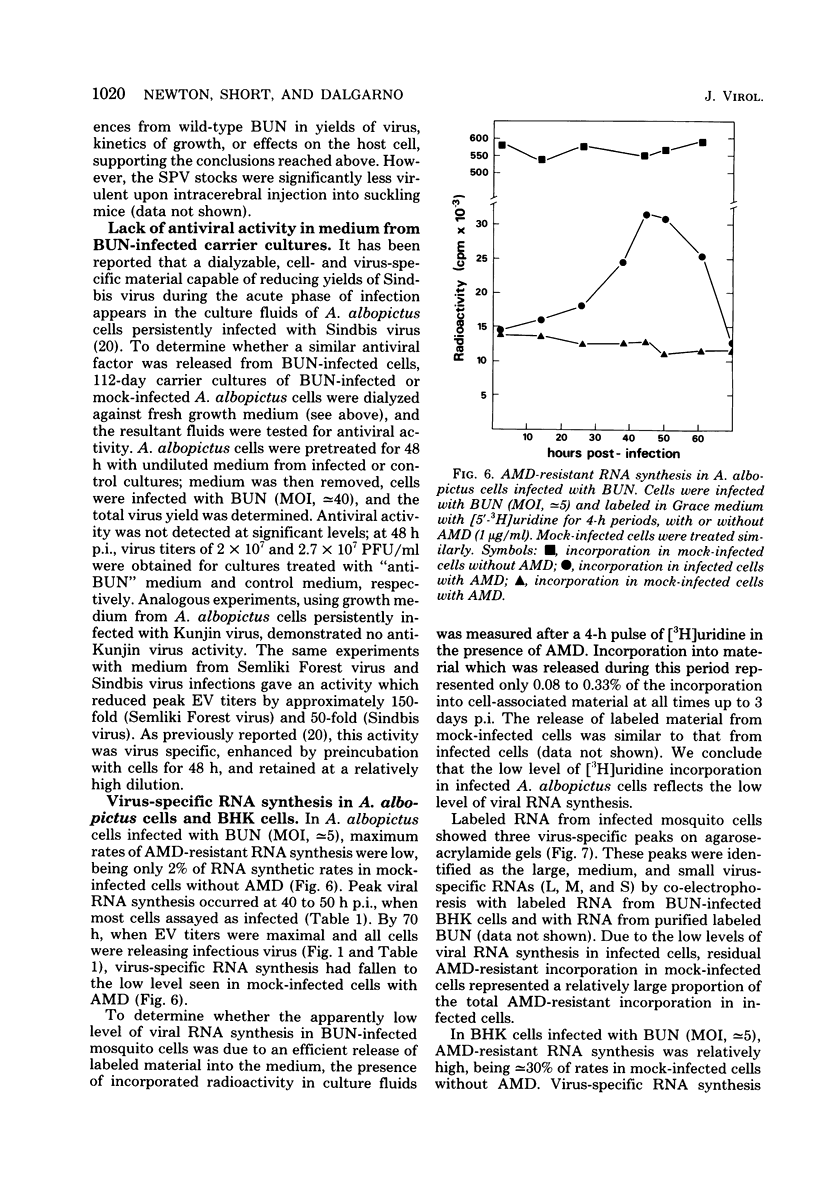
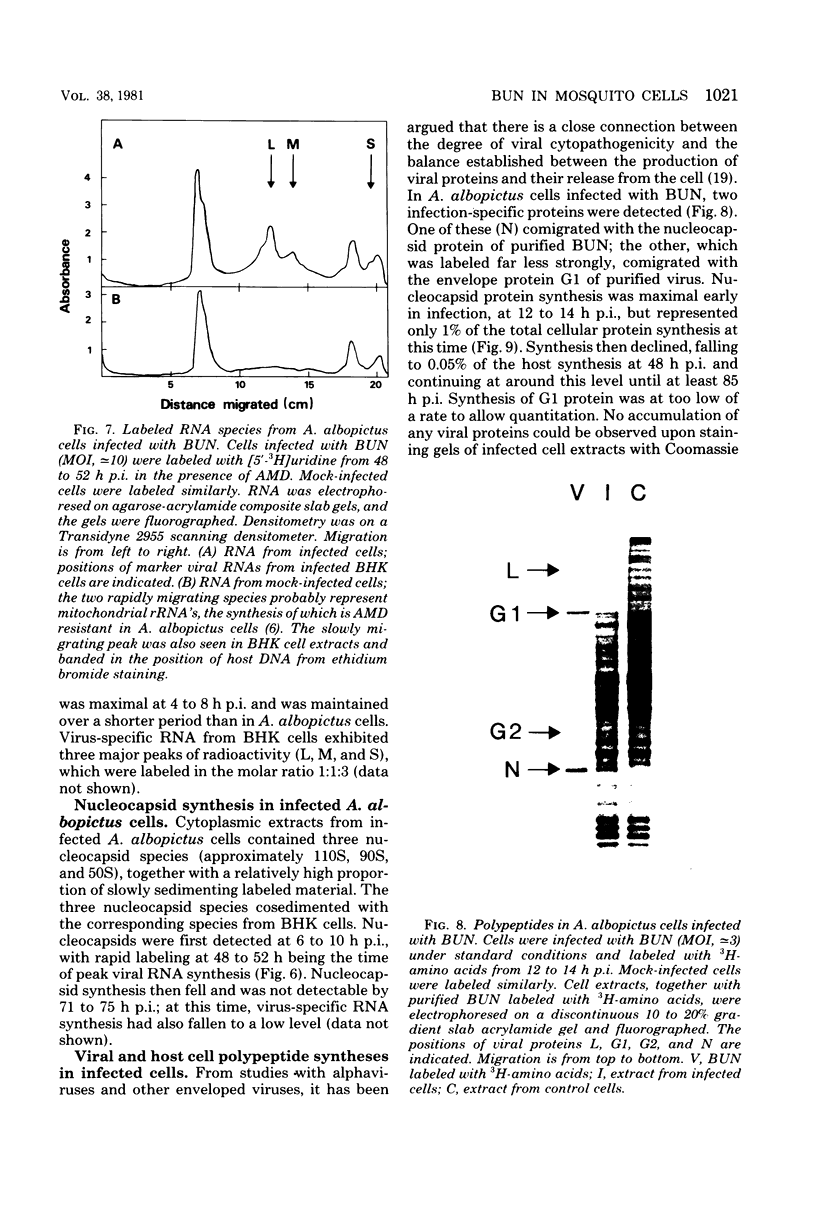
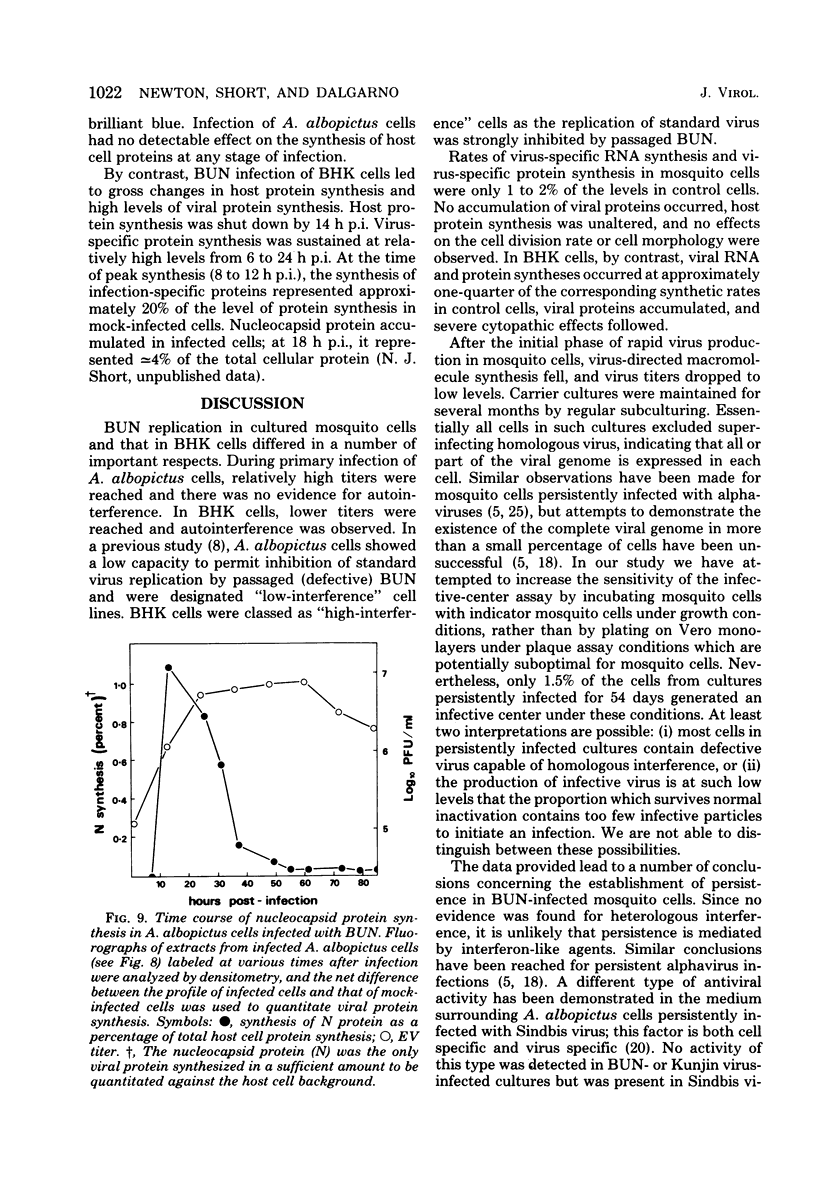
Bunyamwera virus replication was examined in Aedes albopictus (mosquito) cell cultures in which a persistent infection is established and in cytopathically infected BHK cells. During primary infection of A. albopictus cells, Bunyamwera virus reached relatively high titers (≃107 PFU/ml), and autointerference was not observed. Three virus-specific RNAs (L, M, and S) and two virion proteins (N and G1) were detected in infected cells. Maximum rates of viral RNA synthesis and viral protein synthesis were extremely low, corresponding to <2% of the synthetic capacities of uninfected control cells. Viral protein synthesis was maximal at 12 h postinfection and was shut down to barely detectable levels at 24 h postinfection. Virus-specific RNA and nucleocapsid syntheses showed similar patterns of change, but later in infection. The proportions of cells able to release a single PFU at 3, 6, and 54 days postinfection were 100, 50, and 1.5%, respectively. Titers fell to 103 to 105 PFU/ml in carrier cultures. Persistently infected cultures were resistant to superinfection with homologous virus but not with heterologous virus. No changes in host cell protein synthesis or other cytopathic effects were observed at any stage of infection. Small-plaque variants of Bunyamwera virus appeared at approximately 7 days postinfection and increased gradually until they were 75 to 95% of the total infectious virus at 66 days postinfection. Temperature-sensitive mutants appeared between 23 and 49 days postinfection. No antiviral activity similar to that reported in A. albopictus cell cultures persistently infected with Sindbis virus (R. Riedel and D. T. Brown, J. Virol. 29: 51-60, 1979) was detected in culture fluids by 3 months after infection. Bunyamwera virus replicated more rapidly in BHK cells than in mosquito cells but reached lower titers. Autointerference occurred at multiplicities of infection of ≃10. Virus-specific RNA and protein syntheses were at least 20% of the levels in uninfected control cells. Host cell protein synthesis was completely shut down, and nucleocapsid protein accumulated until it was 4% of the total cell protein. We discuss these results in relation to possible mechanisms involved in determining the outcome of arbovirus infection of vertebrate and mosquito cells.
Full text
PDF


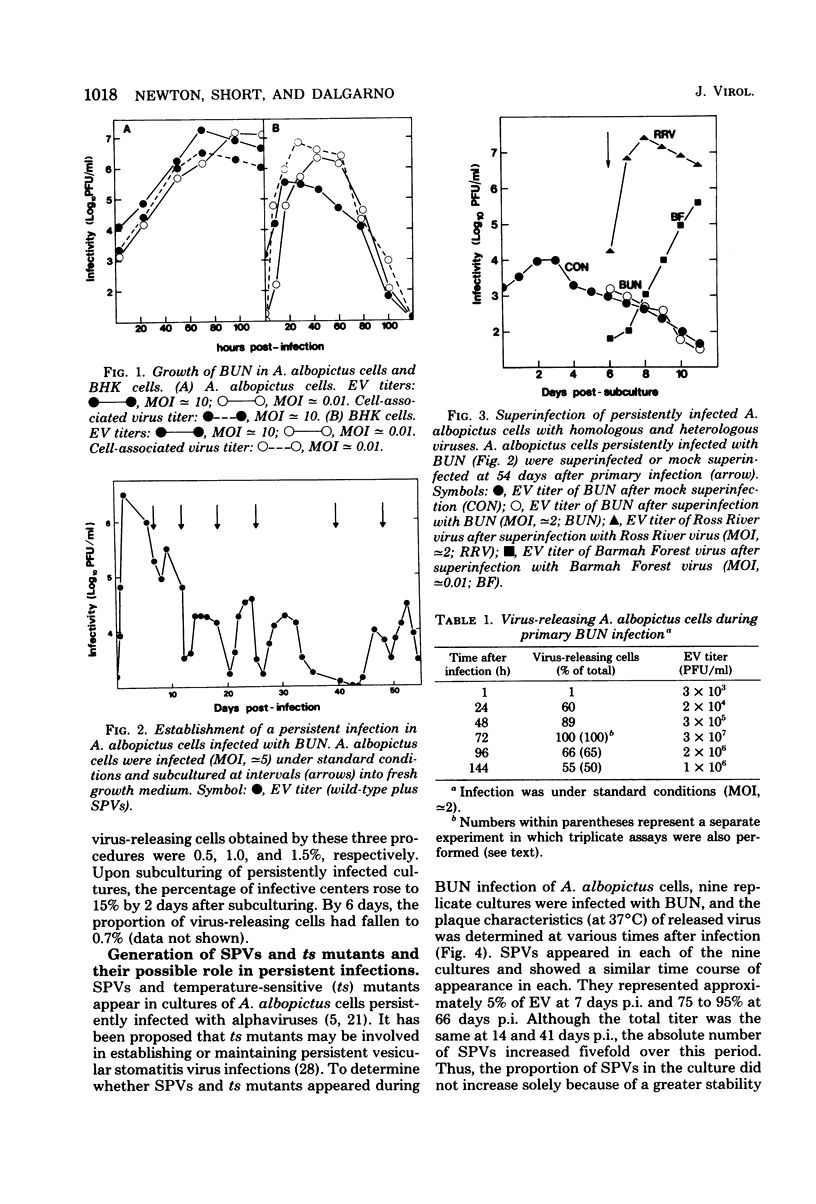
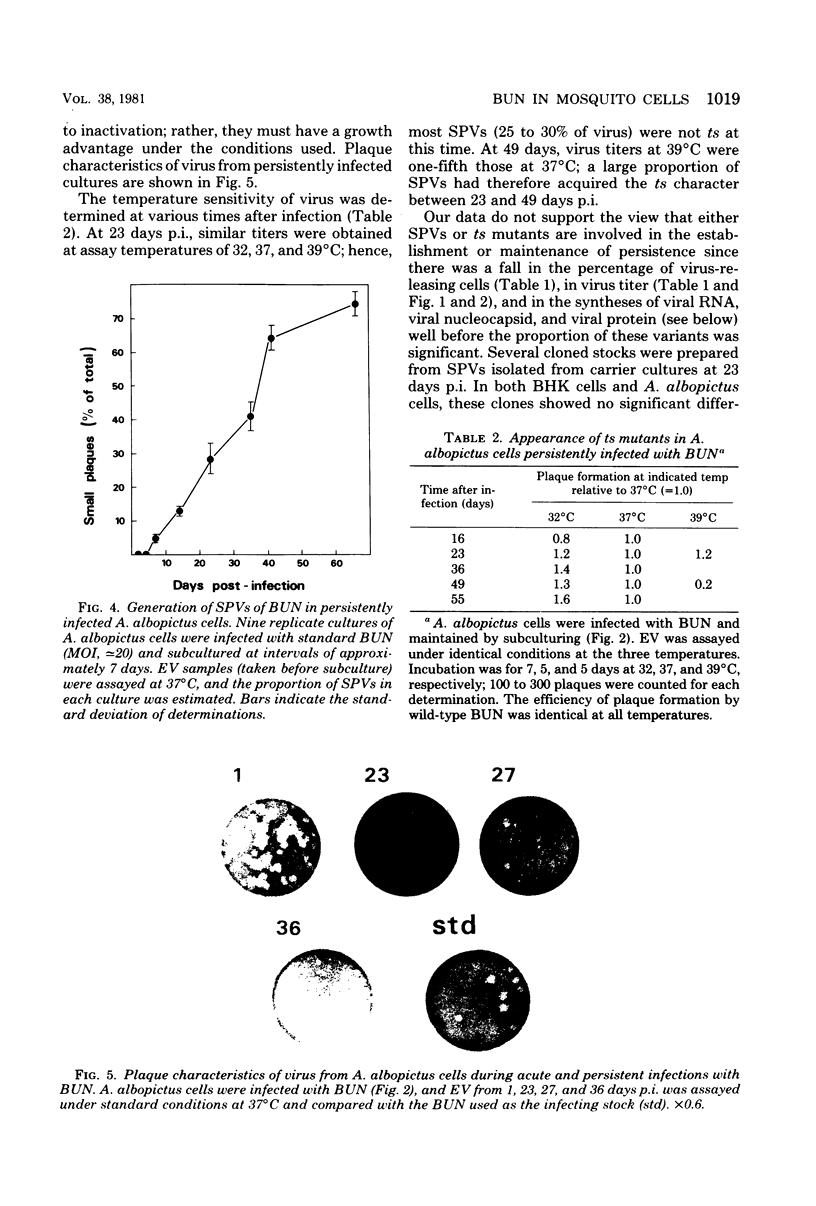


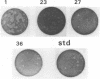
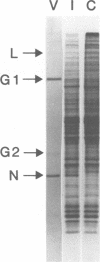
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckley S. M. Susceptibility of the Aedes albopictus and A. aegypti cell lines to infection with arboviruses. Proc Soc Exp Biol Med. 1969 Jun;131(2):625–630. doi: 10.3181/00379727-131-33940. [DOI] [PubMed] [Google Scholar]
- Davey M. W., Dalgarno L. Semliki Forest virus replication in cultured Aedes albopictus cells: studies on the establishment of persistence. J Gen Virol. 1974 Sep;24(3):453–463. doi: 10.1099/0022-1317-24-3-453. [DOI] [PubMed] [Google Scholar]
- Eaton B. T., Randlett D. J. Origin of the actinomycin D insensitive RNA species in Aedes albopictus cells. Nucleic Acids Res. 1978 Apr;5(4):1301–1314. doi: 10.1093/nar/5.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRACE T. D. Establishment of four strains of cells from insect tissues grown in vitro. Nature. 1962 Aug 25;195:788–789. doi: 10.1038/195788a0. [DOI] [PubMed] [Google Scholar]
- Kascsak R. J., Lyons M. J. Bunyamwera virus. II. The generation and nature of defective interfering particles. Virology. 1978 Sep;89(2):539–546. doi: 10.1016/0042-6822(78)90195-2. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lyons M. J., Heyduk J. Aspects of the developmental morphology of California encephalitis virus in cultured vertebrae and arthropod cells and in mouse brain. Virology. 1973 Jul;54(1):37–52. doi: 10.1016/0042-6822(73)90112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhoul Z. Susceptibility of Anopheles gambiae mosquito cell line (MOS 55) to some arboviruses. Acta Virol. 1973 Nov;17(6):507–509. [PubMed] [Google Scholar]
- Martin J. H., Weir R. C., Dalgarno L. Replication of standard and defective Ross River virus in BHK cells: patterns of viral RNA and polypeptide synthesis. Arch Virol. 1979;61(1-2):87–103. doi: 10.1007/BF01320594. [DOI] [PubMed] [Google Scholar]
- Ng M. L., Westaway E. G. Proteins specified by togaviruses in infected Aedes albopictus (Singh) mosquito cells. J Gen Virol. 1979 Apr;43(1):91–101. doi: 10.1099/0022-1317-43-1-91. [DOI] [PubMed] [Google Scholar]
- Obijeski J. F., Bishop D. H., Palmer E. L., Murphy F. A. Segmented genome and nucleocapsid of La Crosse virus. J Virol. 1976 Dec;20(3):664–675. doi: 10.1128/jvi.20.3.664-675.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijeski J. F., Murphy F. A. Bunyaviridae: recent biochemical developments. J Gen Virol. 1977 Oct;37(1):1–14. doi: 10.1099/0022-1317-37-1-1. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Richardson M. A., Boulton R. W., Raghow R. S., Dalgarno L. Polypeptide synthesis in alphavirus-infected Aedes albopictus cells during the establishment of persistent infection. Arch Virol. 1980;63(3-4):263–274. doi: 10.1007/BF01315032. [DOI] [PubMed] [Google Scholar]
- Riedel B., Brown D. T. Novel antiviral activity found in the media of Sindbis virus-persistently infected mosquito (Aedes albopictus) cell cultures. J Virol. 1979 Jan;29(1):51–60. doi: 10.1128/jvi.29.1.51-60.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Koshelnyk K. A., Stollar V. Temperature-sensitive virus from Aedes albopictus cells chronically infected with Sindbis virus. J Virol. 1974 Feb;13(2):439–447. doi: 10.1128/jvi.13.2.439-447.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Occurrence of heat-dissociable ribosomal RNA in insects: the presence of three polynucleotide chains in 26 S RNA from cultured Aedes aegypti cells. J Mol Biol. 1973 Mar 25;75(1):57–72. doi: 10.1016/0022-2836(73)90528-7. [DOI] [PubMed] [Google Scholar]
- Stollar V., Shenk T. E. Homologous viral interference in Aedes albopictus cultures chronically infected with Sindbis virus. J Virol. 1973 Apr;11(4):592–595. doi: 10.1128/jvi.11.4.592-595.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh R. B., Gubler D. J. Laboratory studies of transovarial transmission of La Crosse and other arboviruses by Aedes albopictus and Culex fatigans. Am J Trop Med Hyg. 1975 Sep;24(5):876–880. doi: 10.4269/ajtmh.1975.24.876. [DOI] [PubMed] [Google Scholar]
- Westaway E. G., Reedman B. M. Proteins of the group B arbovirus Kunjin. J Virol. 1969 Nov;4(5):688–693. doi: 10.1128/jvi.4.5.688-693.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Dubovi E. J., Quagliana D. O., Kelly M., Preble O. T. Role of temperature-sensitive mutants in persistent infections initiated with vesicular stomatitis virus. J Virol. 1976 Jul;19(1):90–101. doi: 10.1128/jvi.19.1.90-101.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]