Abstract
Leber hereditary optic neuropathy (LHON) is a type of blindness caused by mtDNA mutations. Three LHON mtDNA mutations at nucleotide positions 3460, 11778, and 14484 are specific for LHON and account for 90% of worldwide cases and are thus designated as "primary" LHON mutations. Fifteen other "secondary" LHON mtDNA mutations have been identified, but their pathogenicity is unclear. mtDNA haplotype and phylogenetic analysis of the primary LHON mutations in North American Caucasian patients and controls has shown that, unlike the 3460 and 11778 mutations, which are distributed throughout the European-derived (Caucasian) mtDNA phylogeny, patients containing the 14484 mutation tended to be associated with European mtDNA haplotype J. To investigate this apparent clustering, we performed chi2-based statistical analyses to compare the distribution of LHON patients on the Caucasian phylogenetic tree. Our results indicate that, unlike the 3460 and 11778 mutations, the 14484 mutation was not distributed on the phylogeny in proportion to the frequencies of the major Caucasian mtDNA haplogroups found in North America. The 14484 mutation was next shown to occur on the haplogroup J background more frequently that expected, consistent with the observation that approximately 75% of worldwide 14484-positive LHON patients occur in association with haplogroup J. The 11778 mutation also exhibited a moderate clustering on haplogroup J. These observations were supported by statistical analysis using all available mutation frequencies reported in the literature. This paper thus illustrates the potential importance of genetic background in certain mtDNA-based diseases, speculates on a pathogenic role for a subset of LHON secondary mutations and their interaction with primary mutations, and provides support for a polygenic model for LHON expression in some cases.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
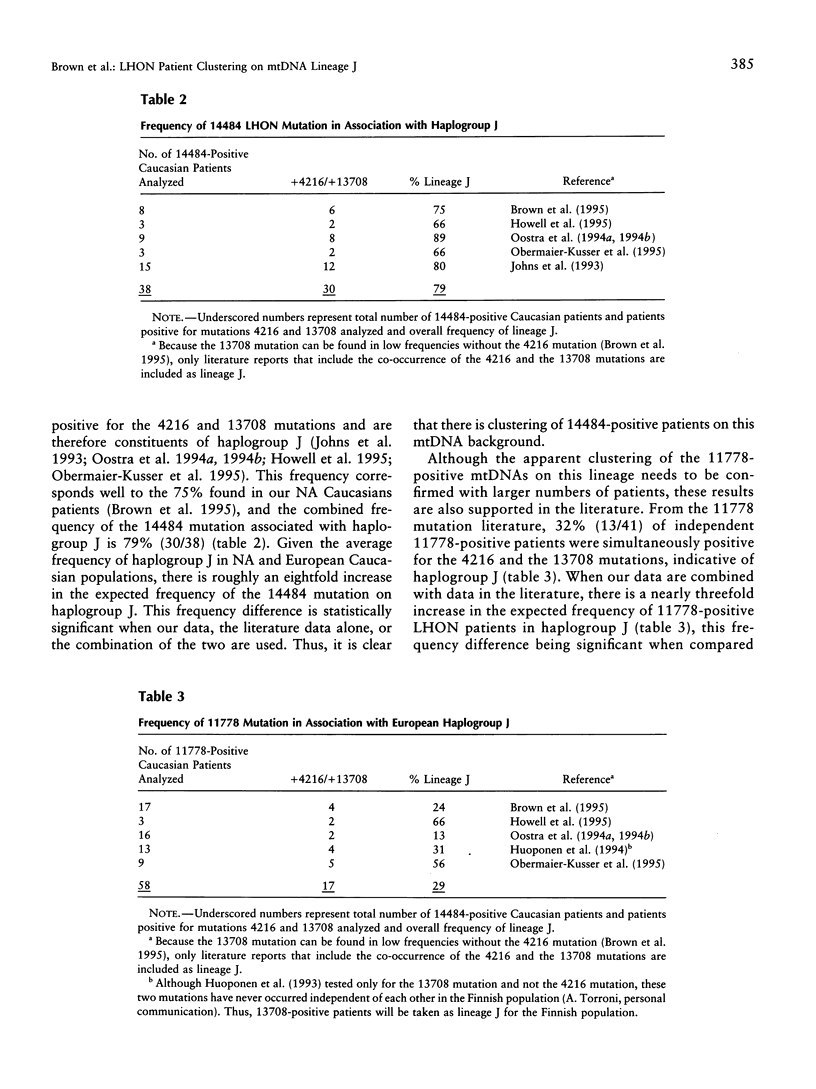
- Brown M. D., Torroni A., Reckord C. L., Wallace D. C. Phylogenetic analysis of Leber's hereditary optic neuropathy mitochondrial DNA's indicates multiple independent occurrences of the common mutations. Hum Mutat. 1995;6(4):311–325. doi: 10.1002/humu.1380060405. [DOI] [PubMed] [Google Scholar]
- Brown M. D., Voljavec A. S., Lott M. T., Torroni A., Yang C. C., Wallace D. C. Mitochondrial DNA complex I and III mutations associated with Leber's hereditary optic neuropathy. Genetics. 1992 Jan;130(1):163–173. doi: 10.1093/genetics/130.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding A. E., Sweeney M. G., Govan G. G., Riordan-Eva P. Pedigree analysis in Leber hereditary optic neuropathy families with a pathogenic mtDNA mutation. Am J Hum Genet. 1995 Jul;57(1):77–86. [PMC free article] [PubMed] [Google Scholar]
- Howell N., Kubacka I., Halvorson S., Howell B., McCullough D. A., Mackey D. Phylogenetic analysis of the mitochondrial genomes from Leber hereditary optic neuropathy pedigrees. Genetics. 1995 May;140(1):285–302. doi: 10.1093/genetics/140.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huoponen K., Lamminen T., Juvonen V., Aula P., Nikoskelainen E., Savontaus M. L. The spectrum of mitochondrial DNA mutations in families with Leber hereditary optic neuroretinopathy. Hum Genet. 1993 Oct;92(4):379–384. doi: 10.1007/BF01247339. [DOI] [PubMed] [Google Scholar]
- Johns D. R., Berman J. Alternative, simultaneous complex I mitochondrial DNA mutations in Leber's hereditary optic neuropathy. Biochem Biophys Res Commun. 1991 Feb 14;174(3):1324–1330. doi: 10.1016/0006-291x(91)91567-v. [DOI] [PubMed] [Google Scholar]
- Johns D. R., Heher K. L., Miller N. R., Smith K. H. Leber's hereditary optic neuropathy. Clinical manifestations of the 14484 mutation. Arch Ophthalmol. 1993 Apr;111(4):495–498. doi: 10.1001/archopht.1993.01090040087038. [DOI] [PubMed] [Google Scholar]
- Johns D. R., Neufeld M. J., Park R. D. An ND-6 mitochondrial DNA mutation associated with Leber hereditary optic neuropathy. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1551–1557. doi: 10.1016/0006-291x(92)90479-5. [DOI] [PubMed] [Google Scholar]
- Mackey D. A., Buttery R. G. Leber hereditary optic neuropathy in Australia. Aust N Z J Ophthalmol. 1992 Aug;20(3):177–184. doi: 10.1111/j.1442-9071.1992.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Newman N. J. Leber's hereditary optic neuropathy. New genetic considerations. Arch Neurol. 1993 May;50(5):540–548. doi: 10.1001/archneur.1993.00540050082021. [DOI] [PubMed] [Google Scholar]
- Obermaier-Kusser B., Lorenz B., Schubring S., Paprotta A., Zerres K., Meitinger T., Meire F., Cochaux P., Blankenagel A., Kommerell G. Features of mtDNA mutation patterns in European pedigrees and sporadic cases with Leber hereditary optic neuropathy. Am J Hum Genet. 1994 Nov;55(5):1063–1066. [PMC free article] [PubMed] [Google Scholar]
- Oostra R. J., Bolhuis P. A., Wijburg F. A., Zorn-Ende G., Bleeker-Wagemakers E. M. Leber's hereditary optic neuropathy: correlations between mitochondrial genotype and visual outcome. J Med Genet. 1994 Apr;31(4):280–286. doi: 10.1136/jmg.31.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra R. J., Bolhuis P. A., Zorn-Ende I., de Kok-Nazaruk M. M., Bleeker-Wagemakers E. M. Leber's hereditary optic neuropathy: no significant evidence for primary or secondary pathogenicity of the 15257 mutation. Hum Genet. 1994 Sep;94(3):265–270. doi: 10.1007/BF00208281. [DOI] [PubMed] [Google Scholar]
- Riordan-Eva P., Sanders M. D., Govan G. G., Sweeney M. G., Da Costa J., Harding A. E. The clinical features of Leber's hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain. 1995 Apr;118(Pt 2):319–337. doi: 10.1093/brain/118.2.319. [DOI] [PubMed] [Google Scholar]
- Sham P. C., Curtis D. Monte Carlo tests for associations between disease and alleles at highly polymorphic loci. Ann Hum Genet. 1995 Jan;59(Pt 1):97–105. doi: 10.1111/j.1469-1809.1995.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Torroni A., Lott M. T., Cabell M. F., Chen Y. S., Lavergne L., Wallace D. C. mtDNA and the origin of Caucasians: identification of ancient Caucasian-specific haplogroups, one of which is prone to a recurrent somatic duplication in the D-loop region. Am J Hum Genet. 1994 Oct;55(4):760–776. [PMC free article] [PubMed] [Google Scholar]


