Abstract
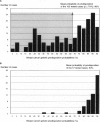
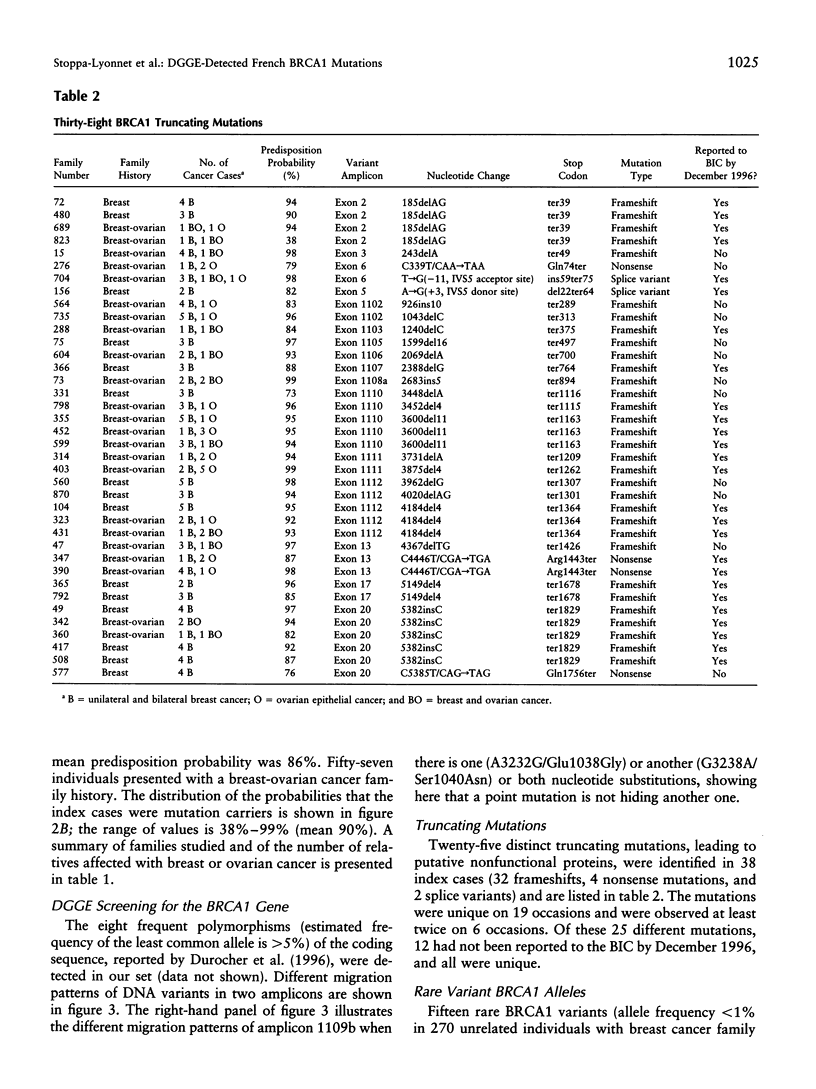
An account of familial aggregation in breast/ovarian cancer has become possible with the identification of BRCA1 germ-line mutations. We evaluated, for 249 individuals registered with the Institut Curie in Paris, the prior probability that an individual carried a mutation that predisposes to these diseases. We chose 160 women for BRCA1 analysis: 103 with a family history of breast cancer and 57 with a family history of breast-ovarian cancer. To detect small mutations, we generated and analyzed 35 overlapping genomic PCR products that cover the coding portion of the gene, by using denaturing gradient gel electrophoresis. Thirty-eight truncating mutations (32 frameshifts, 4 nonsense mutations, and 2 splice variants) were observed in 15% of women with a family history of breast cancer only and in 40% of those with a history of breast-ovarian cancer. Twelve of 25 distinct truncating mutations identified were novel and unique. Most BRCA1 mutations that had been reported more than five times in the Breast Cancer Information Core were present in our series. One mutation (5149del4) observed in two apparently unrelated families most likely originates from a common ancestor. The position of truncating mutations did not significantly affect the ratio of the risk of breast cancer to that of ovarian cancer. In addition, 15 DNA variants (14 missense mutations and 1 neutral mutation) were identified, 9 of which were novel. Indirect evidence suggests that seven of these mutations are deleterious.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel K. J., Xu J., Yin G. Y., Lyons R. H., Meisler M. H., Weber B. L. Mouse Brca1: localization sequence analysis and identification of evolutionarily conserved domains. Hum Mol Genet. 1995 Dec;4(12):2265–2273. doi: 10.1093/hmg/4.12.2265. [DOI] [PubMed] [Google Scholar]
- Bienstock R. J., Darden T., Wiseman R., Pedersen L., Barrett J. C. Molecular modeling of the amino-terminal zinc ring domain of BRCA1. Cancer Res. 1996 Jun 1;56(11):2539–2545. [PubMed] [Google Scholar]
- Callebaut I., Mornon J. P. From BRCA1 to RAP1: a widespread BRCT module closely associated with DNA repair. FEBS Lett. 1997 Jan 2;400(1):25–30. doi: 10.1016/s0014-5793(96)01312-9. [DOI] [PubMed] [Google Scholar]
- Claus E. B., Risch N., Thompson W. D. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet. 1991 Feb;48(2):232–242. [PMC free article] [PubMed] [Google Scholar]
- Couch F. J., Weber B. L. Mutations and polymorphisms in the familial early-onset breast cancer (BRCA1) gene. Breast Cancer Information Core. Hum Mutat. 1996;8(1):8–18. doi: 10.1002/humu.1380080102. [DOI] [PubMed] [Google Scholar]
- Durocher F., Shattuck-Eidens D., McClure M., Labrie F., Skolnick M. H., Goldgar D. E., Simard J. Comparison of BRCA1 polymorphisms, rare sequence variants and/or missense mutations in unaffected and breast/ovarian cancer populations. Hum Mol Genet. 1996 Jun;5(6):835–842. doi: 10.1093/hmg/5.6.835. [DOI] [PubMed] [Google Scholar]
- Easton D. F., Bishop D. T., Ford D., Crockford G. P. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993 Apr;52(4):678–701. [PMC free article] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1579–1583. doi: 10.1073/pnas.80.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D., Easton D. F. The genetics of breast and ovarian cancer. Br J Cancer. 1995 Oct;72(4):805–812. doi: 10.1038/bjc.1995.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L. S., Ostermeyer E. A., Szabo C. I., Dowd P., Lynch E. D., Rowell S. E., King M. C. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet. 1994 Dec;8(4):399–404. doi: 10.1038/ng1294-399. [DOI] [PubMed] [Google Scholar]
- Futreal P. A., Liu Q., Shattuck-Eidens D., Cochran C., Harshman K., Tavtigian S., Bennett L. M., Haugen-Strano A., Swensen J., Miki Y. BRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994 Oct 7;266(5182):120–122. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- Gayther S. A., Warren W., Mazoyer S., Russell P. A., Harrington P. A., Chiano M., Seal S., Hamoudi R., van Rensburg E. J., Dunning A. M. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat Genet. 1995 Dec;11(4):428–433. doi: 10.1038/ng1295-428. [DOI] [PubMed] [Google Scholar]
- Grompe M. The rapid detection of unknown mutations in nucleic acids. Nat Genet. 1993 Oct;5(2):111–117. doi: 10.1038/ng1093-111. [DOI] [PubMed] [Google Scholar]
- Hall J. M., Lee M. K., Newman B., Morrow J. E., Anderson L. A., Huey B., King M. C. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990 Dec 21;250(4988):1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- Hamelin R., Jego N., Laurent-Puig P., Vidaud M., Thomas G. Efficient screening of p53 mutations by denaturing gradient gel electrophoresis in colorectal tumors. Oncogene. 1993 Aug;8(8):2213–2220. [PubMed] [Google Scholar]
- Koonin E. V., Altschul S. F., Bork P. BRCA1 protein products ... Functional motifs... Nat Genet. 1996 Jul;13(3):266–268. doi: 10.1038/ng0796-266. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M. Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet. 1984 Mar;36(2):460–465. [PMC free article] [PubMed] [Google Scholar]
- Miki Y., Swensen J., Shattuck-Eidens D., Futreal P. A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L. M., Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994 Oct 7;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Moyret C., Theillet C., Puig P. L., Molés J. P., Thomas G., Hamelin R. Relative efficiency of denaturing gradient gel electrophoresis and single strand conformation polymorphism in the detection of mutations in exons 5 to 8 of the p53 gene. Oncogene. 1994 Jun;9(6):1739–1743. [PubMed] [Google Scholar]
- Narod S. A., Ford D., Devilee P., Barkardottir R. B., Lynch H. T., Smith S. A., Ponder B. A., Weber B. L., Garber J. E., Birch J. M. An evaluation of genetic heterogeneity in 145 breast-ovarian cancer families. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995 Jan;56(1):254–264. [PMC free article] [PubMed] [Google Scholar]
- Neuhausen S. L., Mazoyer S., Friedman L., Stratton M., Offit K., Caligo A., Tomlinson G., Cannon-Albright L., Bishop T., Kelsell D. Haplotype and phenotype analysis of six recurrent BRCA1 mutations in 61 families: results of an international study. Am J Hum Genet. 1996 Feb;58(2):271–280. [PMC free article] [PubMed] [Google Scholar]
- Peelen T., Cornelis R. S., van Vliet M., Petrij-Bosch A., Cleton-Jansen A. M., Meijers-Heijboer H., Klijn J. G., Vasen H. F., Cornelisse C. J., Devilee P. The majority of 22 Dutch high-risk breast cancer families are due to either BRCA1 or BRCA2. Eur J Hum Genet. 1996;4(4):225–230. doi: 10.1159/000472203. [DOI] [PubMed] [Google Scholar]
- Scully R., Chen J., Plug A., Xiao Y., Weaver D., Feunteun J., Ashley T., Livingston D. M. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997 Jan 24;88(2):265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- Serova O., Montagna M., Torchard D., Narod S. A., Tonin P., Sylla B., Lynch H. T., Feunteun J., Lenoir G. M. A high incidence of BRCA1 mutations in 20 breast-ovarian cancer families. Am J Hum Genet. 1996 Jan;58(1):42–51. [PMC free article] [PubMed] [Google Scholar]
- Shattuck-Eidens D., McClure M., Simard J., Labrie F., Narod S., Couch F., Hoskins K., Weber B., Castilla L., Erdos M. A collaborative survey of 80 mutations in the BRCA1 breast and ovarian cancer susceptibility gene. Implications for presymptomatic testing and screening. JAMA. 1995 Feb 15;273(7):535–541. [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. M., Lee M. K., Szabo C. I., Jerome N., McEuen M., Taylor M., Hood L., King M. C. Complete genomic sequence and analysis of 117 kb of human DNA containing the gene BRCA1. Genome Res. 1996 Nov;6(11):1029–1049. doi: 10.1101/gr.6.11.1029. [DOI] [PubMed] [Google Scholar]
- Sobol H., Birnbaum D., Eisinger F. Evidence for a third breast-cancer susceptibility gene. Lancet. 1994 Oct 22;344(8930):1151–1152. doi: 10.1016/s0140-6736(94)90655-6. [DOI] [PubMed] [Google Scholar]
- Tavtigian S. V., Simard J., Rommens J., Couch F., Shattuck-Eidens D., Neuhausen S., Merajver S., Thorlacius S., Offit K., Stoppa-Lyonnet D. The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat Genet. 1996 Mar;12(3):333–337. doi: 10.1038/ng0396-333. [DOI] [PubMed] [Google Scholar]
- Wooster R., Bignell G., Lancaster J., Swift S., Seal S., Mangion J., Collins N., Gregory S., Gumbs C., Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995 Dec 21;378(6559):789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- Wooster R., Neuhausen S. L., Mangion J., Quirk Y., Ford D., Collins N., Nguyen K., Seal S., Tran T., Averill D. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994 Sep 30;265(5181):2088–2090. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]
- Wu L. C., Wang Z. W., Tsan J. T., Spillman M. A., Phung A., Xu X. L., Yang M. C., Hwang L. Y., Bowcock A. M., Baer R. Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat Genet. 1996 Dec;14(4):430–440. doi: 10.1038/ng1296-430. [DOI] [PubMed] [Google Scholar]