Abstract
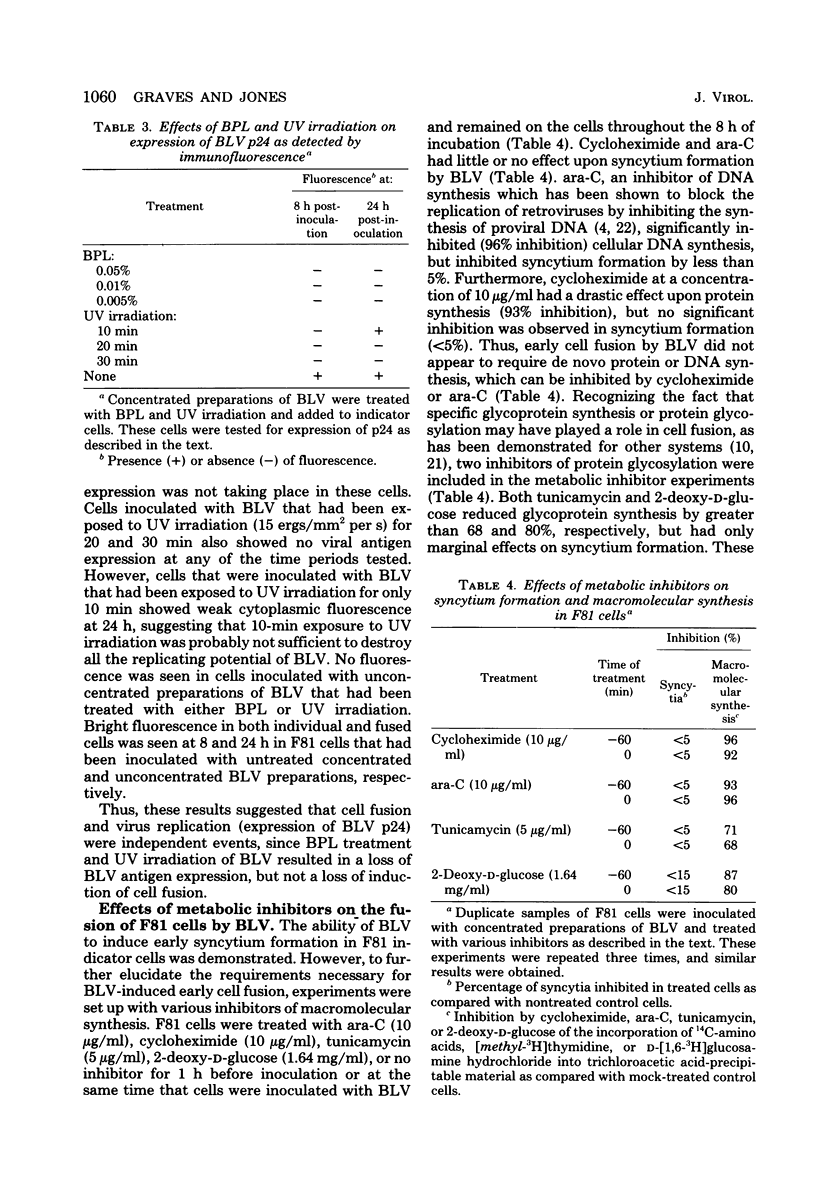
Bovine leukemia virus (BLV) from either persistently infected bat cells or fetal lamb kidney cells induced rapid syncytium formation in F81 indicator cells. Distinct syncytia were seen within 2 h after inoculation of cells with highly concentrated (500-fold) cell-free BLV preparations and within 4 to 8 h when unconcentrated cell-free BLV preparations were used. Indicator cell densities of 1 × 105 to 2 × 105 were optimal for rapid and maximal syncytium formation. Pretreatment of BLV with reference BLV leukemic serum and antiserum prepared against purified BLV significantly inhibited (95%) syncytium formation. Reference bovine viral diarrhea virus serum, foamy-like bovine syncytial virus serum, and control serum had little effect (17% inhibition). Antiserum to BLV gp51 inhibited syncytium formation by greater than 96%, whereas antiserum to BLV p24 reduced syncytium activity to a much lesser extent (38% inhibition). Treatment of BLV with β-propiolactone (0.005 to 0.05%) had little or no effect upon syncytium-forming activity, whereas UV irradiation (15 ergs/mm2 per s for 30 min) reduced, but did not completely destroy, the fusion activity. However, both β-propiolactone and UV irradiation drastically reduced the replication potential of BLV, as demonstrated by the lack of p24 expression in the inoculated cells. Concentrations of cycloheximide, cytosine arabinoside, tunicamycin, and 2-deoxy-D-glucose which effectively blocked cellular macromolecular synthesis did not significantly inhibit syncytium formation. These latter results suggested that de novo protein and DNA synthesis as well as protein glycosylation were not required for early syncytium formation. Thus, these experiments demonstrated that replication of BLV by the indicator cells was not essential for cell fusion.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton C. V., Soria A. E., Gilden R. V. Direct syncytial assay for the quantitation of bovine leukemia virus. Infect Immun. 1978 Apr;20(1):307–309. doi: 10.1128/iai.20.1.307-309.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Varmus H. E., Stavnezer E., Hunter E., Vogt P. K. Biological and biochemical studies on the inactivation of avian oncoviruses by ultraviolet irradiation. Virology. 1977 Apr;77(2):689–704. doi: 10.1016/0042-6822(77)90492-5. [DOI] [PubMed] [Google Scholar]
- Bratt M. A., Gallaher W. R. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):536–543. doi: 10.1073/pnas.64.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Hunter E. The characterization of Mason-Pfizer monkey virus-induced cell fusion. Virology. 1979 Jun;95(2):421–433. doi: 10.1016/0042-6822(79)90497-5. [DOI] [PubMed] [Google Scholar]
- Damsky C. H., Levy-Benshimol A., Buck C. A., Warren L. Effect of tunicamycin on the synthesis, intracellular transport and shedding of membrane glycoproteins in BHK cells. Exp Cell Res. 1979 Mar 1;119(1):1–13. doi: 10.1016/0014-4827(79)90329-x. [DOI] [PubMed] [Google Scholar]
- Diglio C. A., Ferrer J. F. Induction of syncytia by the bovine C-type leukemia virus. Cancer Res. 1976 Mar;36(3):1056–1067. [PubMed] [Google Scholar]
- Ferrer J. F., Cabradilla C. D. The phenomenon of polykaryocytosis induced by BLV in mixed cultures: specificity, mechanisms and application to the diagnosis of BLV infection in cattle. Ann Rech Vet. 1978;9(4):721–728. [PubMed] [Google Scholar]
- Fischinger P. J., Blevins C. S., Nomura S. Simple, quantitative assay for both xenotropic murine leukemia and ecotropic feline leukemia viruses. J Virol. 1974 Jul;14(1):177–179. doi: 10.1128/jvi.14.1.177-179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Peebles P. T., Nomura S., Haapala D. K. Isolation of RD-114-like oncornavirus from a cat cell line. J Virol. 1973 Jun;11(6):978–985. doi: 10.1128/jvi.11.6.978-985.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher W. R., Levitan D. B., Blough H. A. Effect of 2-deoxy-D-glucose on cell fusion induced by Newcastle disease and herpes simplex viruses. Virology. 1973 Sep;55(1):193–201. doi: 10.1016/s0042-6822(73)81021-9. [DOI] [PubMed] [Google Scholar]
- Ghysdael J., Kettmann R., Burny A. Translation of bovine leukemia virus virion RNAs in heterologous protein-synthesizing systems. J Virol. 1979 Mar;29(3):1087–1098. doi: 10.1128/jvi.29.3.1087-1098.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves D. C., Ferrer J. F. In vitro transmission and propagation of the bovine leukemia virus in monolayer cell cultures. Cancer Res. 1976 Nov;36(11 Pt 1):4152–4159. [PubMed] [Google Scholar]
- Guillemain B., Mamoun R., Levy D., Astier T., Irgens K., Parodi A. A. Early polykaryocytosis inhibition: a simple quantitative in vitro assay for the detection of bovine leukemia virus infection in cattle. Eur J Cancer. 1978 Aug;14(8):811–827. doi: 10.1016/0014-2964(78)90097-x. [DOI] [PubMed] [Google Scholar]
- Hirschman S. Z., Funke V. Effect of diethylaminoethyl-dextran on the replication of a murine sarcoma (Moloney)-leukemia virus complex in mouse embryo cultures (38577). Proc Soc Exp Biol Med. 1975 Feb;148(2):527–531. doi: 10.3181/00379727-148-38577. [DOI] [PubMed] [Google Scholar]
- Irgens K., Pinelli C., Guillemain B., Levy D., Parodi A. L. Early syncytium formation induced by bovine leukemia virus in mixed cultures. Biomedicine. 1977 Mar;27(2):49–50. [PubMed] [Google Scholar]
- Jerabek L., Gupta P., Ferrer J. F. An infectivity assay for the bovine leukemia virus based on the induction of the major internal virion antigen in susceptible cell cultures. Ann Rech Vet. 1978;9(4):729–734. [PubMed] [Google Scholar]
- Ogura H., Paulsen J., Bauer H. Cross-neutralization of ovine and bovine C-type leukemia virus-induced syncytia formation. Cancer Res. 1977 May;37(5):1486–1489. [PubMed] [Google Scholar]
- Ogura H. XC cell fusion by murine leukemia viruses: fusion from without. Med Microbiol Immunol. 1976 Dec 1;162(3-4):175–181. doi: 10.1007/BF02120995. [DOI] [PubMed] [Google Scholar]
- Poste G. Mechanisms of virus-induced cell fusion. Int Rev Cytol. 1972;33:157–252. doi: 10.1016/s0074-7696(08)61451-5. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Takehara M. Effect of certain inhibitors of glycoprotein synthesis on cell fusion induced by vesicular stomatitis virus. Microbiol Immunol. 1979;23(3):167–176. doi: 10.1111/j.1348-0421.1979.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Garfin D. E., Levinson W. E., Bishop J. M., Goodman H. M. Tumor virus ribonucleic acid directed deoxyribonucleic acid synthesis: nucleotide sequence at the 5' terminus of nascent deoxyribonucleic acid. Biochemistry. 1974 Jul 16;13(15):3159–3163. doi: 10.1021/bi00712a024. [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969 Jul;38(3):414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- Wainberg M. A., Hjorth R. N., Howe C. Effect of -propiolactone on Sendai virus. Appl Microbiol. 1971 Oct;22(4):618–621. doi: 10.1128/am.22.4.618-621.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., Yuen P. H., Kaufman S. J. Induction of syncytia by Moloney murine leukemia virus in myoblasts defective in differentiation. J Virol. 1977 Jan;21(1):319–327. doi: 10.1128/jvi.21.1.319-327.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling D. A., Keshet I. Fusion activity of virions of murine leukemia virus. Virology. 1979 May;95(1):185–196. doi: 10.1016/0042-6822(79)90413-6. [DOI] [PubMed] [Google Scholar]




