Abstract
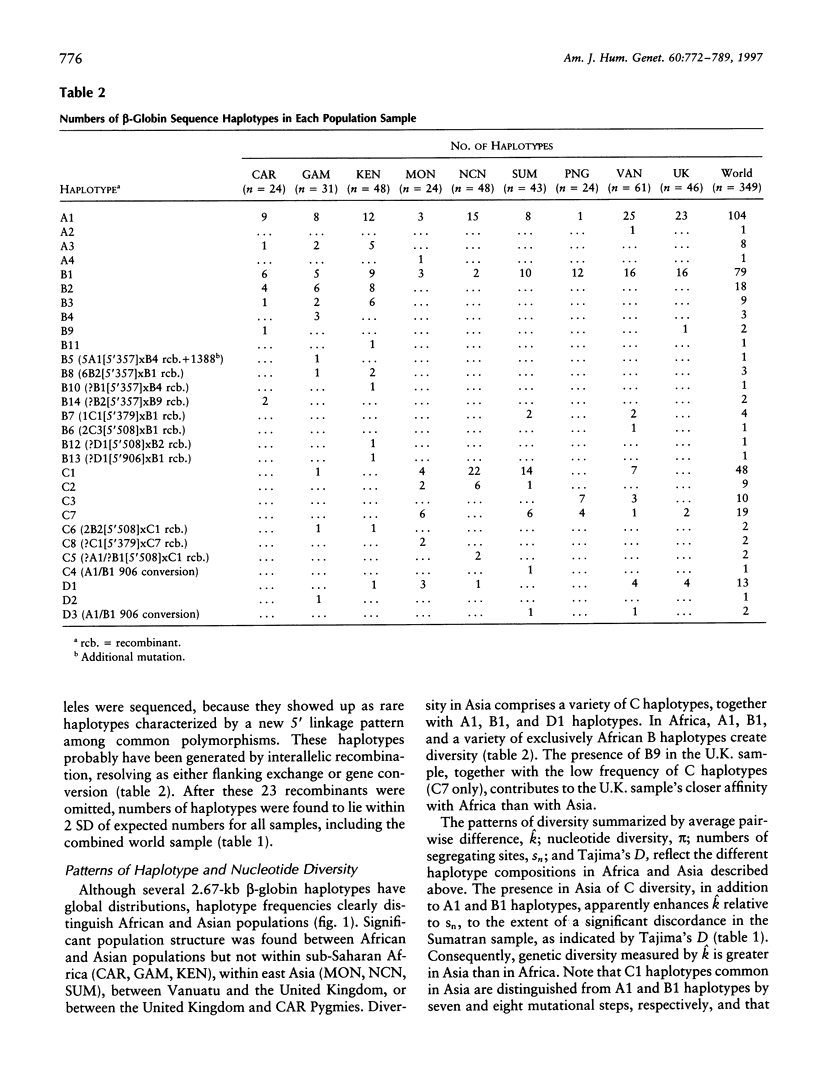
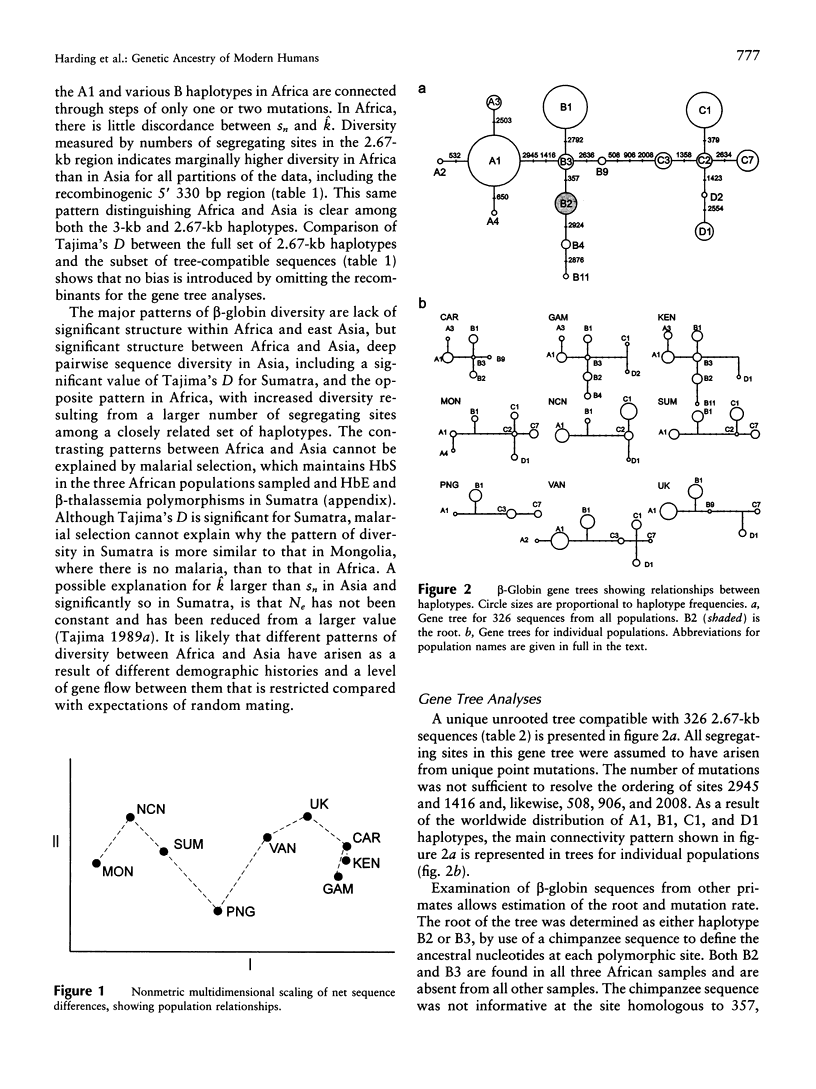
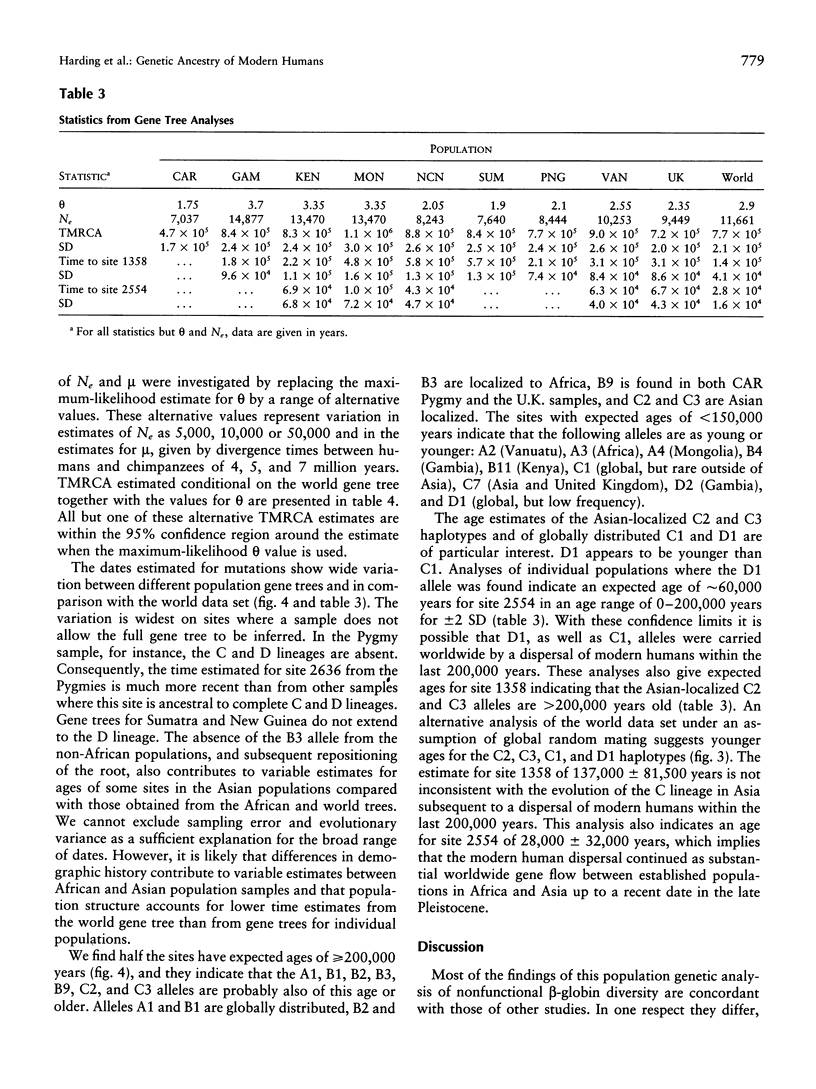
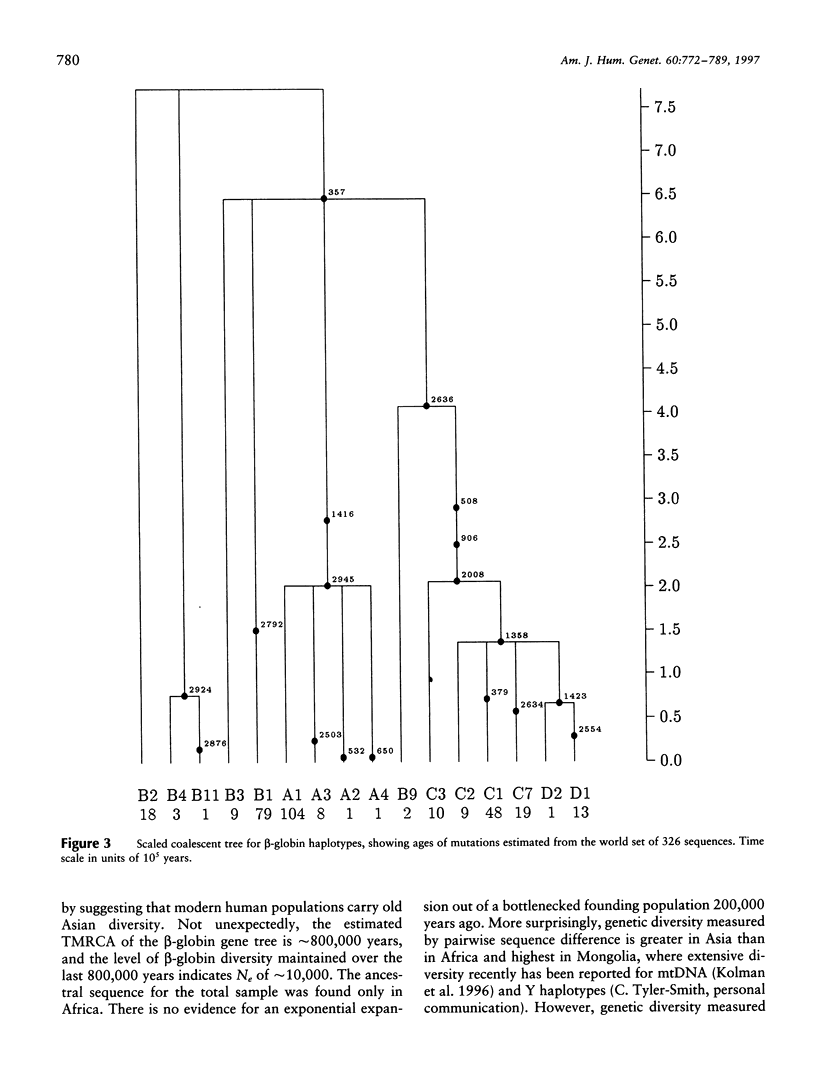
A 3-kb region encompassing the beta-globin gene has been analyzed for allelic sequence polymorphism in nine populations from Africa, Asia, and Europe. A unique gene tree was constructed from 326 sequences of 349 in the total sample. New maximum-likelihood methods for analyzing gene trees on the basis of coalescence theory have been used. The most recent common ancestor of the beta-globin gene tree is a sequence found only in Africa and estimated to have arisen approximately 800,000 years ago. There is no evidence for an exponential expansion out of a bottlenecked founding population, and an effective population size of approximately 10,000 has been maintained. Modest differences in levels of beta-globin diversity between Africa and Asia are better explained by greater African effective population size than by greater time depth. There may have been a reduction of Asian effective population size in recent evolutionary history. Characteristically Asian ancestry is estimated to be older than 200,000 years, suggesting that the ancestral hominid population at this time was widely dispersed across Africa and Asia. Patterns of beta-globin diversity suggest extensive worldwide late Pleistocene gene flow and are not easily reconciled with a unidirectional migration out of Africa 100,000 years ago and total replacement of archaic populations in Asia.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C. Protection afforded by sickle-cell trait against subtertian malareal infection. Br Med J. 1954 Feb 6;1(4857):290–294. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis S. E., Boehm C. D., Giardina P. J., Kazazian H. H., Jr Nonrandom association of polymorphic restriction sites in the beta-globin gene cluster. Proc Natl Acad Sci U S A. 1982 Jan;79(1):137–141. doi: 10.1073/pnas.79.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour J. A., Anttinen T., May C. A., Vega E. E., Sajantila A., Kidd J. R., Kidd K. K., Bertranpetit J., Päbo S., Jeffreys A. J. Minisatellite diversity supports a recent African origin for modern humans. Nat Genet. 1996 Jun;13(2):154–160. doi: 10.1038/ng0696-154. [DOI] [PubMed] [Google Scholar]
- Bowcock A. M., Ruiz-Linares A., Tomfohrde J., Minch E., Kidd J. R., Cavalli-Sforza L. L. High resolution of human evolutionary trees with polymorphic microsatellites. Nature. 1994 Mar 31;368(6470):455–457. doi: 10.1038/368455a0. [DOI] [PubMed] [Google Scholar]
- Brookfield J. F. Human evolution. A new molecular view of human origins. Curr Biol. 1994 Jul 1;4(7):651–652. doi: 10.1016/s0960-9822(00)00145-7. [DOI] [PubMed] [Google Scholar]
- Chen Y. S., Torroni A., Excoffier L., Santachiara-Benerecetti A. S., Wallace D. C. Analysis of mtDNA variation in African populations reveals the most ancient of all human continent-specific haplogroups. Am J Hum Genet. 1995 Jul;57(1):133–149. [PMC free article] [PubMed] [Google Scholar]
- Donnelly P., Tavaré S. Coalescents and genealogical structure under neutrality. Annu Rev Genet. 1995;29:401–421. doi: 10.1146/annurev.ge.29.120195.002153. [DOI] [PubMed] [Google Scholar]
- Dorit R. L., Akashi H., Gilbert W. Absence of polymorphism at the ZFY locus on the human Y chromosome. Science. 1995 May 26;268(5214):1183–1185. doi: 10.1126/science.7761836. [DOI] [PubMed] [Google Scholar]
- Ewens W. J. The sampling theory of selectively neutral alleles. Theor Popul Biol. 1972 Mar;3(1):87–112. doi: 10.1016/0040-5809(72)90035-4. [DOI] [PubMed] [Google Scholar]
- Flint J., Harding R. M., Clegg J. B., Boyce A. J. Why are some genetic diseases common? Distinguishing selection from other processes by molecular analysis of globin gene variants. Hum Genet. 1993 Mar;91(2):91–117. doi: 10.1007/BF00222709. [DOI] [PubMed] [Google Scholar]
- Hammer M. F. A recent common ancestry for human Y chromosomes. Nature. 1995 Nov 23;378(6555):376–378. doi: 10.1038/378376a0. [DOI] [PubMed] [Google Scholar]
- Hill A. V., Allsopp C. E., Kwiatkowski D., Taylor T. E., Yates S. N., Anstey N. M., Wirima J. J., Brewster D. R., McMichael A. J., Molyneux M. E. Extensive genetic diversity in the HLA class II region of Africans, with a focally predominant allele, DRB1*1304. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2277–2281. doi: 10.1073/pnas.89.6.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson R. R., Boos D. D., Kaplan N. L. A statistical test for detecting geographic subdivision. Mol Biol Evol. 1992 Jan;9(1):138–151. doi: 10.1093/oxfordjournals.molbev.a040703. [DOI] [PubMed] [Google Scholar]
- Hudson R. R. Properties of a neutral allele model with intragenic recombination. Theor Popul Biol. 1983 Apr;23(2):183–201. doi: 10.1016/0040-5809(83)90013-8. [DOI] [PubMed] [Google Scholar]
- Jorde L. B., Bamshad M. J., Watkins W. S., Zenger R., Fraley A. E., Krakowiak P. A., Carpenter K. D., Soodyall H., Jenkins T., Rogers A. R. Origins and affinities of modern humans: a comparison of mitochondrial and nuclear genetic data. Am J Hum Genet. 1995 Sep;57(3):523–538. doi: 10.1002/ajmg.1320570340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolman C. J., Sambuughin N., Bermingham E. Mitochondrial DNA analysis of Mongolian populations and implications for the origin of New World founders. Genetics. 1996 Apr;142(4):1321–1334. doi: 10.1093/genetics/142.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitman M. Nucleotide polymorphism at the alcohol dehydrogenase locus of Drosophila melanogaster. Nature. 1983 Aug 4;304(5925):412–417. doi: 10.1038/304412a0. [DOI] [PubMed] [Google Scholar]
- Li W. H., Sadler L. A. Low nucleotide diversity in man. Genetics. 1991 Oct;129(2):513–523. doi: 10.1093/genetics/129.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson J. J., Excoffier L., Swinburn C., Boyce A. J., Harding R. M., Langaney A., Clegg J. B. High diversity of alpha-globin haplotypes in a Senegalese population, including many previously unreported variants. Am J Hum Genet. 1995 Nov;57(5):1186–1198. [PMC free article] [PubMed] [Google Scholar]
- Mountain J. L., Cavalli-Sforza L. L. Inference of human evolution through cladistic analysis of nuclear DNA restriction polymorphisms. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6515–6519. doi: 10.1073/pnas.91.14.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Takezaki N. The root of the phylogenetic tree of human populations. Mol Biol Evol. 1996 Jan;13(1):170–177. doi: 10.1093/oxfordjournals.molbev.a025553. [DOI] [PubMed] [Google Scholar]
- Newton C. R., Graham A., Heptinstall L. E., Powell S. J., Summers C., Kalsheker N., Smith J. C., Markham A. F. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 1989 Apr 11;17(7):2503–2516. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Goff S. C., Boehm C. D., Sexton J. P., Waber P. G., Giardina P. J. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982 Apr 15;296(5858):627–631. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr The mutation and polymorphism of the human beta-globin gene and its surrounding DNA. Annu Rev Genet. 1984;18:131–171. doi: 10.1146/annurev.ge.18.120184.001023. [DOI] [PubMed] [Google Scholar]
- Ruvolo M., Zehr S., von Dornum M., Pan D., Chang B., Lin J. Mitochondrial COII sequences and modern human origins. Mol Biol Evol. 1993 Nov;10(6):1115–1135. doi: 10.1093/oxfordjournals.molbev.a040068. [DOI] [PubMed] [Google Scholar]
- Sherry S. T., Rogers A. R., Harpending H., Soodyall H., Jenkins T., Stoneking M. Mismatch distributions of mtDNA reveal recent human population expansions. Hum Biol. 1994 Oct;66(5):761–775. [PubMed] [Google Scholar]
- Stringer C. B., Andrews P. Genetic and fossil evidence for the origin of modern humans. Science. 1988 Mar 11;239(4845):1263–1268. doi: 10.1126/science.3125610. [DOI] [PubMed] [Google Scholar]
- Strobeck C., Morgan K. The effect of intragenic recombination on the number of alleles in a finite population. Genetics. 1978 Apr;88(4):829–844. doi: 10.1093/genetics/88.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima F. Evolutionary relationship of DNA sequences in finite populations. Genetics. 1983 Oct;105(2):437–460. doi: 10.1093/genetics/105.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata N., Satta Y., Klein J. Divergence time and population size in the lineage leading to modern humans. Theor Popul Biol. 1995 Oct;48(2):198–221. doi: 10.1006/tpbi.1995.1026. [DOI] [PubMed] [Google Scholar]
- Tishkoff S. A., Dietzsch E., Speed W., Pakstis A. J., Kidd J. R., Cheung K., Bonné-Tamir B., Santachiara-Benerecetti A. S., Moral P., Krings M. Global patterns of linkage disequilibrium at the CD4 locus and modern human origins. Science. 1996 Mar 8;271(5254):1380–1387. doi: 10.1126/science.271.5254.1380. [DOI] [PubMed] [Google Scholar]
- Trabuchet G., Elion J., Baudot G., Pagnier J., Bouhass R., Nigon V. M., Labie D., Krishnamoorthy R. Origin and spread of beta-globin gene mutations in India, Africa, and Mediterranea: analysis of the 5' flanking and intragenic sequences of beta S and beta C genes. Hum Biol. 1991 Jun;63(3):241–252. [PubMed] [Google Scholar]
- Vigilant L., Stoneking M., Harpending H., Hawkes K., Wilson A. C. African populations and the evolution of human mitochondrial DNA. Science. 1991 Sep 27;253(5027):1503–1507. doi: 10.1126/science.1840702. [DOI] [PubMed] [Google Scholar]
- Wainscoat J. S., Hill A. V., Boyce A. L., Flint J., Hernandez M., Thein S. L., Old J. M., Lynch J. R., Falusi A. G., Weatherall D. J. Evolutionary relationships of human populations from an analysis of nuclear DNA polymorphisms. Nature. 1986 Feb 6;319(6053):491–493. doi: 10.1038/319491a0. [DOI] [PubMed] [Google Scholar]
- Ward R. H., Frazier B. L., Dew-Jager K., Päbo S. Extensive mitochondrial diversity within a single Amerindian tribe. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8720–8724. doi: 10.1073/pnas.88.19.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]


