Abstract
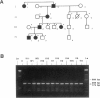
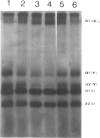
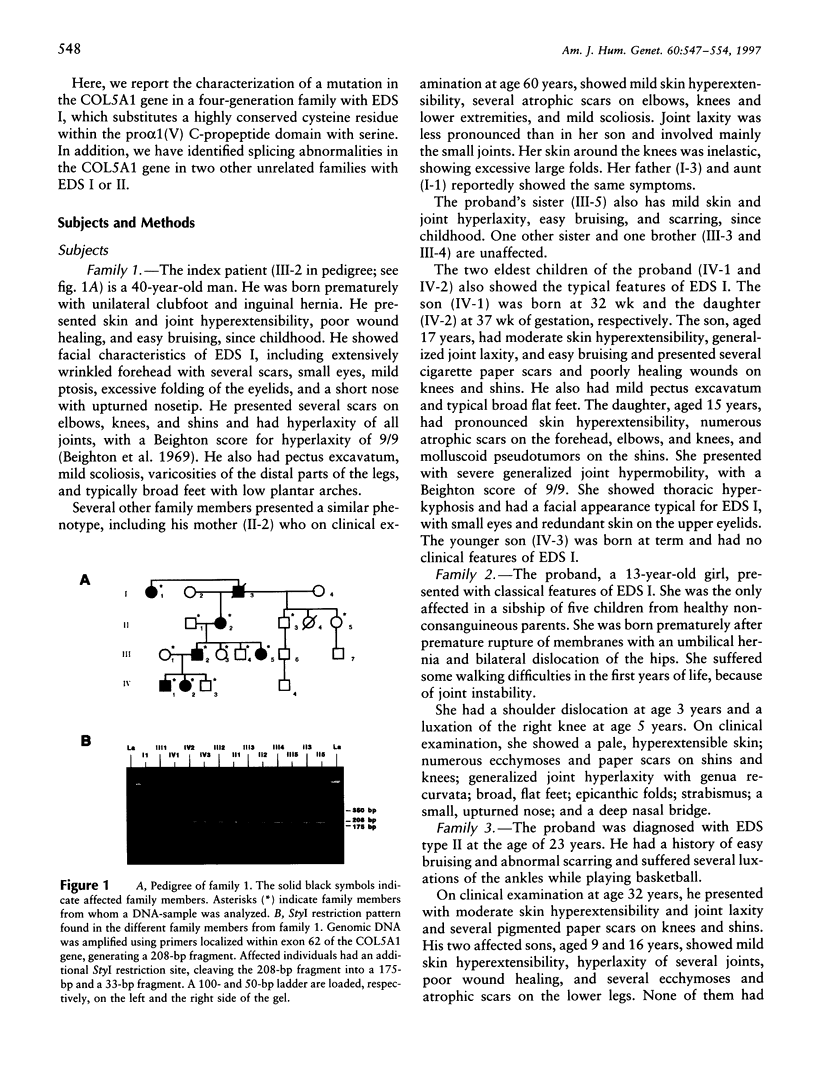
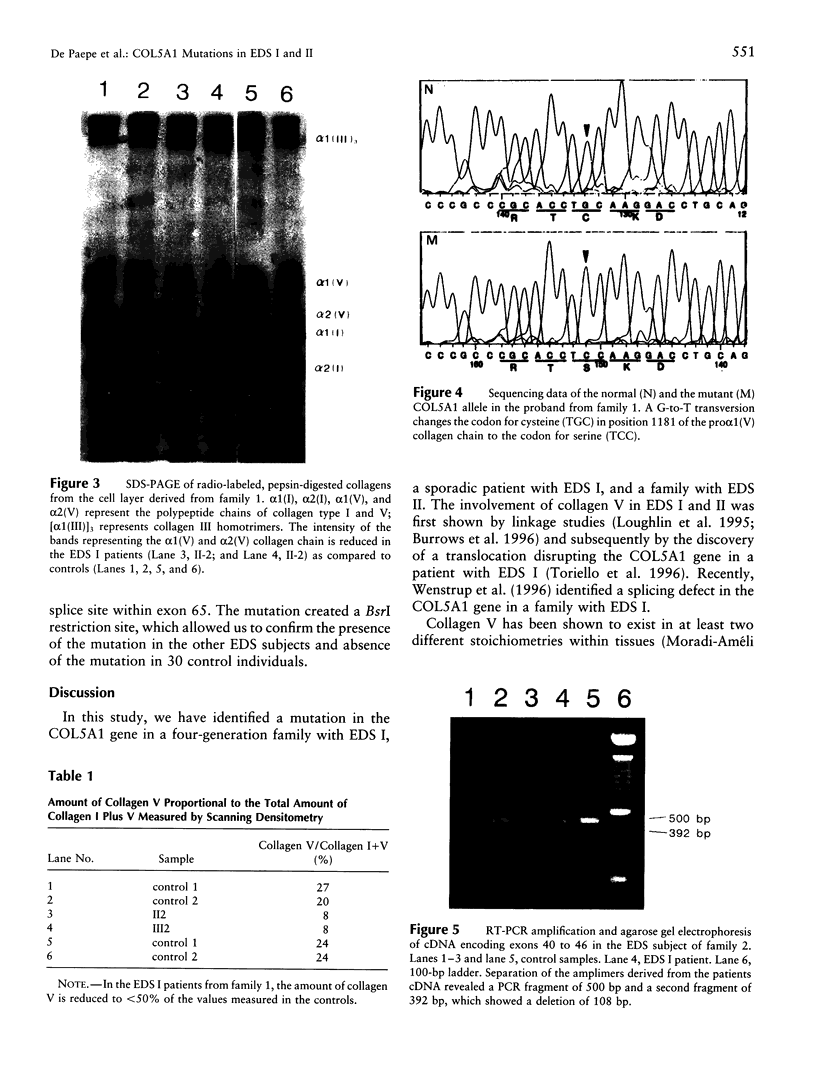
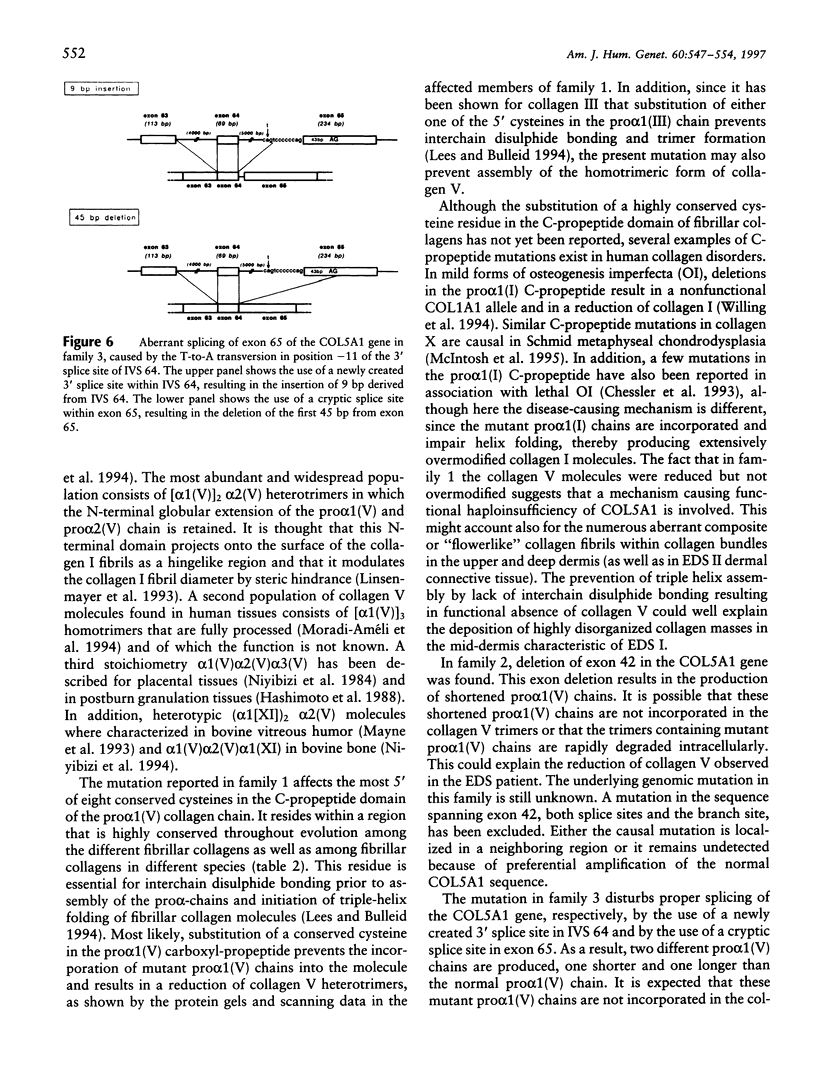
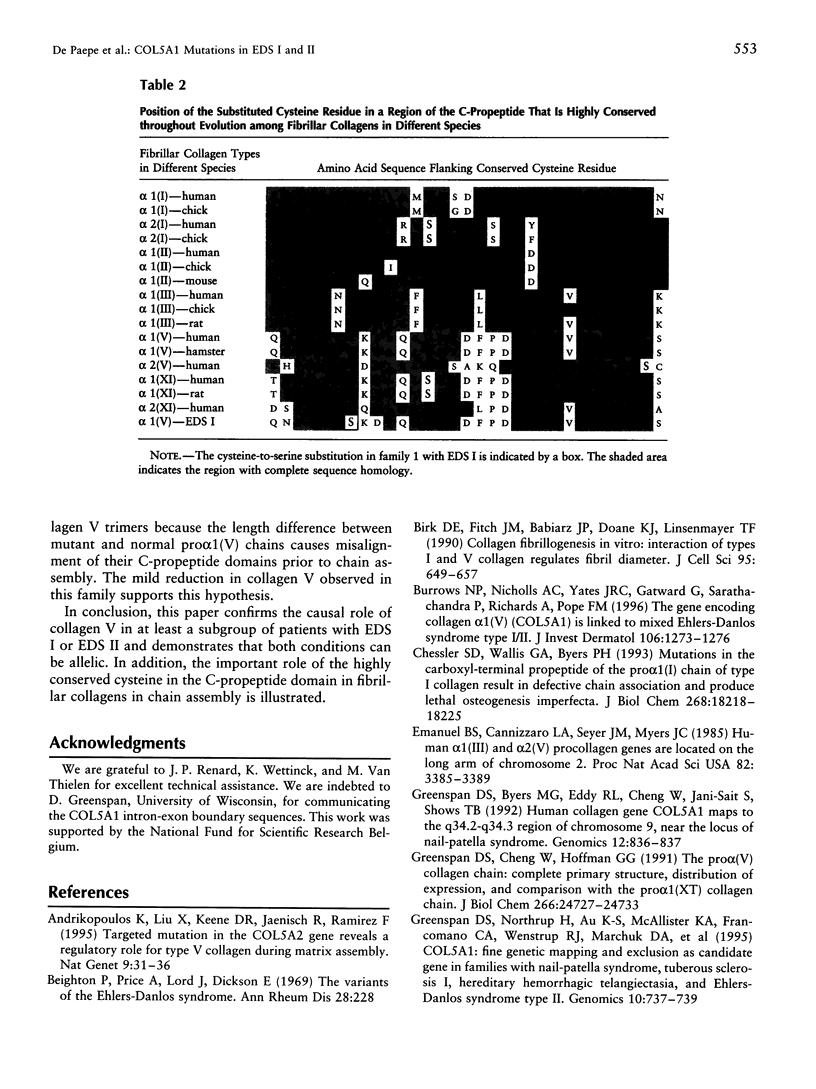
The Ehlers-Danlos syndrome (EDS) is a heterogeneous connective-tissue disorder of which at least nine subtypes are recognized. Considerable clinical overlap exists between the EDS I and II subtypes, suggesting that both are allelic disorders. Recent evidence based on linkage and transgenic mice studies suggest that collagen V is causally involved in human EDS. Collagen V forms heterotypic fibrils with collagen I in many tissues and plays an important role in collagen I fibrillogenesis. We have identified a mutation in COL5A1, the gene encoding the pro(alpha)1(V) collagen chain, segregating with EDS I in a four-generation family. The mutation causes the substitution of the most 5' cysteine residue by a serine within a highly conserved sequence of the pro(alpha)1(V) C-propeptide domain and causes reduction of collagen V by preventing incorporation of the mutant pro(alpha)1(V) chains in the collagen V trimers. In addition, we have detected splicing defects in the COL5A1 gene in a patient with EDS I and in a family with EDS II. These findings confirm the causal role of collagen V in at least a subgroup of EDS I, prove that EDS I and II are allelic conditions, and represent a, so far, unique example of a human collagen disorder caused by substitution of a highly conserved cysteine residue in the C-propeptide domain of a fibrillar collagen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrikopoulos K., Liu X., Keene D. R., Jaenisch R., Ramirez F. Targeted mutation in the col5a2 gene reveals a regulatory role for type V collagen during matrix assembly. Nat Genet. 1995 Jan;9(1):31–36. doi: 10.1038/ng0195-31. [DOI] [PubMed] [Google Scholar]
- Ball M. A., McCullough J. L., Weinstein G. D. Percutaneous absorption of methotrexate: effect on epidermal DNA synthesis in hairless mice. J Invest Dermatol. 1982 Jul;79(1):7–10. doi: 10.1111/1523-1747.ep12510415. [DOI] [PubMed] [Google Scholar]
- Beighton P., Price A., Lord J., Dickson E. Variants of the Ehlers-Danlos syndrome. Clinical, biochemical, haematological, and chromosomal features of 100 patients. Ann Rheum Dis. 1969 May;28(3):228–245. doi: 10.1136/ard.28.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk D. E., Fitch J. M., Babiarz J. P., Doane K. J., Linsenmayer T. F. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990 Apr;95(Pt 4):649–657. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- Burrows N. P., Nicholls A. C., Yates J. R., Gatward G., Sarathachandra P., Richards A., Pope F. M. The gene encoding collagen alpha1(V)(COL5A1) is linked to mixed Ehlers-Danlos syndrome type I/II. J Invest Dermatol. 1996 Jun;106(6):1273–1276. doi: 10.1111/1523-1747.ep12348978. [DOI] [PubMed] [Google Scholar]
- Chessler S. D., Wallis G. A., Byers P. H. Mutations in the carboxyl-terminal propeptide of the pro alpha 1(I) chain of type I collagen result in defective chain association and produce lethal osteogenesis imperfecta. J Biol Chem. 1993 Aug 25;268(24):18218–18225. [PubMed] [Google Scholar]
- Emanuel B. S., Cannizzaro L. A., Seyer J. M., Myers J. C. Human alpha 1(III) and alpha 2(V) procollagen genes are located on the long arm of chromosome 2. Proc Natl Acad Sci U S A. 1985 May;82(10):3385–3389. doi: 10.1073/pnas.82.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan D. S., Byers M. G., Eddy R. L., Cheng W., Jani-Sait S., Shows T. B. Human collagen gene COL5A1 maps to the q34.2----q34.3 region of chromosome 9, near the locus for nail-patella syndrome. Genomics. 1992 Apr;12(4):836–837. doi: 10.1016/0888-7543(92)90320-r. [DOI] [PubMed] [Google Scholar]
- Greenspan D. S., Cheng W., Hoffman G. G. The pro-alpha 1(V) collagen chain. Complete primary structure, distribution of expression, and comparison with the pro-alpha 1(XI) collagen chain. J Biol Chem. 1991 Dec 25;266(36):24727–24733. [PubMed] [Google Scholar]
- Greenspan D. S., Northrup H., Au K. S., McAllister K. A., Francomano C. A., Wenstrup R. J., Marchuk D. A., Kwiatkowski D. J. COL5A1: fine genetic mapping and exclusion as candidate gene in families with nail-patella syndrome, tuberous sclerosis 1, hereditary hemorrhagic telangiectasia, and Ehlers-Danlos Syndrome type II. Genomics. 1995 Feb 10;25(3):737–739. doi: 10.1016/0888-7543(95)80021-d. [DOI] [PubMed] [Google Scholar]
- Greenspan D. S., Pasquinelli A. E. BstUI and DpnII RFLPs at the COL5A1 gene. Hum Mol Genet. 1994 Feb;3(2):385–385. doi: 10.1093/hmg/3.2.385-a. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Kobayashi K., Hoshino T., Aoyama H., Hayakawa T. Distinction between two molecular species of type V collagen from human post-burn granulation tissues. J Invest Dermatol. 1988 Sep;91(3):238–242. doi: 10.1111/1523-1747.ep12470365. [DOI] [PubMed] [Google Scholar]
- Hausser I., Anton-Lamprecht I. Differential ultrastructural aberrations of collagen fibrils in Ehlers-Danlos syndrome types I-IV as a means of diagnostics and classification. Hum Genet. 1994 Apr;93(4):394–407. doi: 10.1007/BF00201664. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lees J. F., Bulleid N. J. The role of cysteine residues in the folding and association of the COOH-terminal propeptide of types I and III procollagen. J Biol Chem. 1994 Sep 30;269(39):24354–24360. [PubMed] [Google Scholar]
- Linsenmayer T. F., Gibney E., Igoe F., Gordon M. K., Fitch J. M., Fessler L. I., Birk D. E. Type V collagen: molecular structure and fibrillar organization of the chicken alpha 1(V) NH2-terminal domain, a putative regulator of corneal fibrillogenesis. J Cell Biol. 1993 Jun;121(5):1181–1189. doi: 10.1083/jcb.121.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin J., Irven C., Hardwick L. J., Butcher S., Walsh S., Wordsworth P., Sykes B. Linkage of the gene that encodes the alpha 1 chain of type V collagen (COL5A1) to type II Ehlers-Danlos syndrome (EDS II). Hum Mol Genet. 1995 Sep;4(9):1649–1651. doi: 10.1093/hmg/4.9.1649. [DOI] [PubMed] [Google Scholar]
- Mayne R., Brewton R. G., Mayne P. M., Baker J. R. Isolation and characterization of the chains of type V/type XI collagen present in bovine vitreous. J Biol Chem. 1993 May 5;268(13):9381–9386. [PubMed] [Google Scholar]
- McIntosh I., Abbott M. H., Francomano C. A. Concentration of mutations causing Schmid metaphyseal chondrodysplasia in the C-terminal noncollagenous domain of type X collagen. Hum Mutat. 1995;5(2):121–125. doi: 10.1002/humu.1380050204. [DOI] [PubMed] [Google Scholar]
- Moradi-Améli M., Rousseau J. C., Kleman J. P., Champliaud M. F., Boutillon M. M., Bernillon J., Wallach J., Van der Rest M. Diversity in the processing events at the N-terminus of type-V collagen. Eur J Biochem. 1994 May 1;221(3):987–995. doi: 10.1111/j.1432-1033.1994.tb18815.x. [DOI] [PubMed] [Google Scholar]
- Niyibizi C., Chan R., Wu J. J., Eyre D. A 92 kDa gelatinase (MMP-9) cleavage site in native type V collagen. Biochem Biophys Res Commun. 1994 Jul 15;202(1):328–333. doi: 10.1006/bbrc.1994.1931. [DOI] [PubMed] [Google Scholar]
- Niyibizi C., Fietzek P. P., van der Rest M. Human placenta type V collagens. Evidence for the existence of an alpha 1(V) alpha 2(V) alpha 3(V) collagen molecule. J Biol Chem. 1984 Nov 25;259(22):14170–14174. [PubMed] [Google Scholar]
- Nuytinck L., Dalgleish R., Spotila L., Renard J. P., Van Regemorter N., De Paepe A. Substitution of glycine-661 by serine in the alpha1(I) and alpha2(I) chains of type I collagen results in different clinical and biochemical phenotypes. Hum Genet. 1996 Mar;97(3):324–329. doi: 10.1007/BF02185764. [DOI] [PubMed] [Google Scholar]
- Sokolov B. P., Prytkov A. N., Tromp G., Knowlton R. G., Prockop D. J. Exclusion of COL1A1, COL1A2, and COL3A1 genes as candidate genes for Ehlers-Danlos syndrome type I in one large family. Hum Genet. 1991 Dec;88(2):125–129. doi: 10.1007/BF00206058. [DOI] [PubMed] [Google Scholar]
- Toriello H. V., Glover T. W., Takahara K., Byers P. H., Miller D. E., Higgins J. V., Greenspan D. S. A translocation interrupts the COL5A1 gene in a patient with Ehlers-Danlos syndrome and hypomelanosis of Ito. Nat Genet. 1996 Jul;13(3):361–365. doi: 10.1038/ng0796-361. [DOI] [PubMed] [Google Scholar]
- Wenstrup R. J., Langland G. T., Willing M. C., D'Souza V. N., Cole W. G. A splice-junction mutation in the region of COL5A1 that codes for the carboxyl propeptide of pro alpha 1(V) chains results in the gravis form of the Ehlers-Danlos syndrome (type I). Hum Mol Genet. 1996 Nov;5(11):1733–1736. doi: 10.1093/hmg/5.11.1733. [DOI] [PubMed] [Google Scholar]
- Willing M. C., Deschenes S. P., Scott D. A., Byers P. H., Slayton R. L., Pitts S. H., Arikat H., Roberts E. J. Osteogenesis imperfecta type I: molecular heterogeneity for COL1A1 null alleles of type I collagen. Am J Hum Genet. 1994 Oct;55(4):638–647. [PMC free article] [PubMed] [Google Scholar]
- Wordsworth B. P., Ogilvie D. J., Sykes B. C. Segregation analysis of the structural genes of the major fibrillar collagens provides further evidence of molecular heterogeneity in type II Ehlers-Danlos syndrome. Br J Rheumatol. 1991 Jun;30(3):173–177. doi: 10.1093/rheumatology/30.3.173. [DOI] [PubMed] [Google Scholar]
- Wordsworth P., Ogilvie D., Smith R., Sykes B. Exclusion of the alpha 1(II) collagen structural gene as the mutant locus in type II Ehlers-Danlos syndrome. Ann Rheum Dis. 1985 Jul;44(7):431–433. doi: 10.1136/ard.44.7.431. [DOI] [PMC free article] [PubMed] [Google Scholar]