Abstract
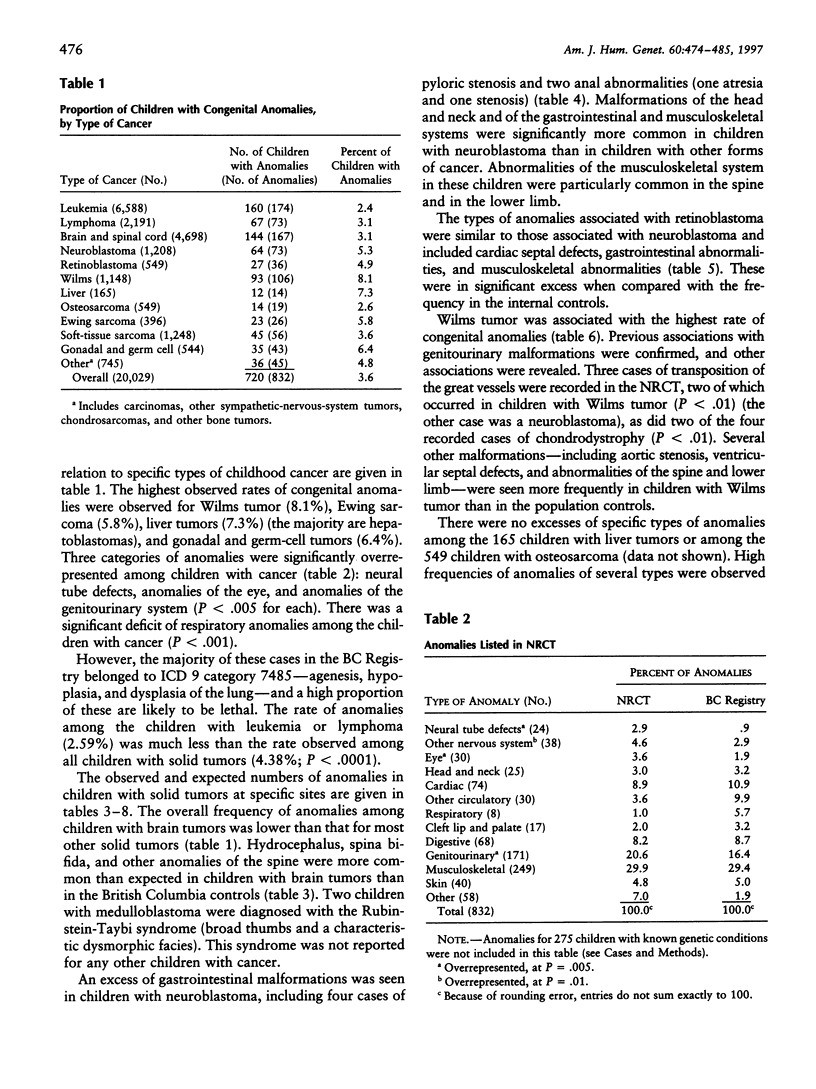
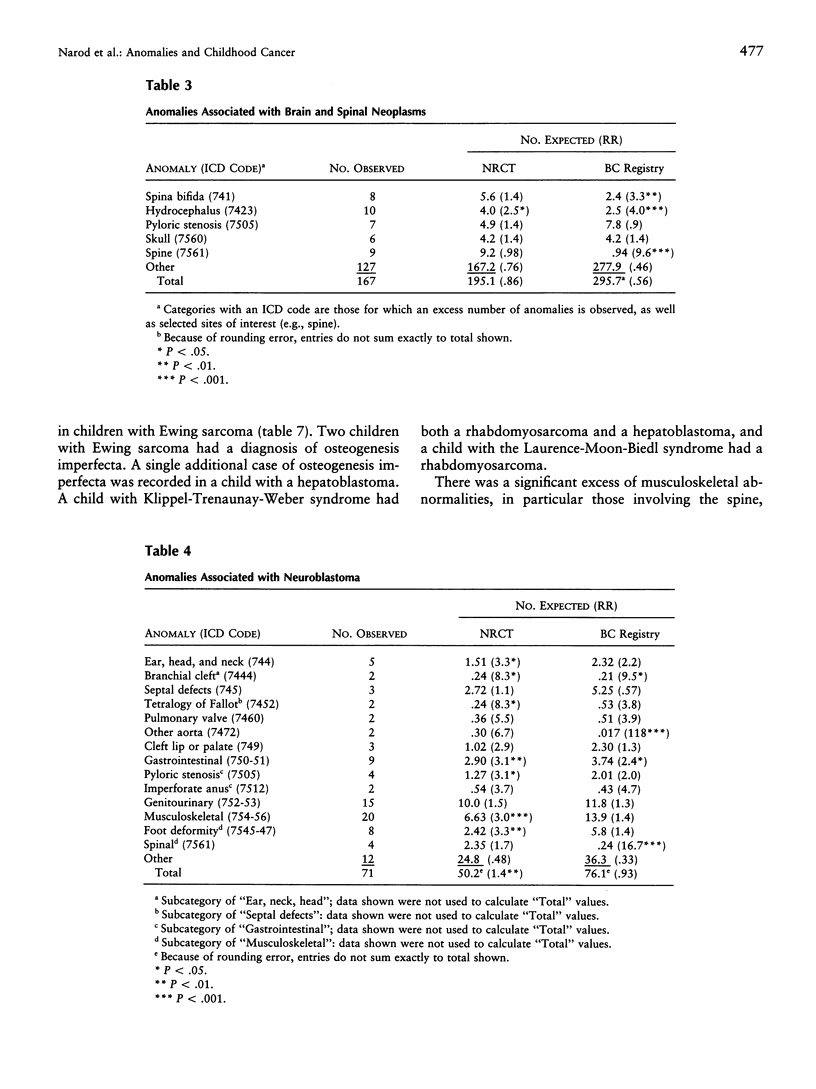
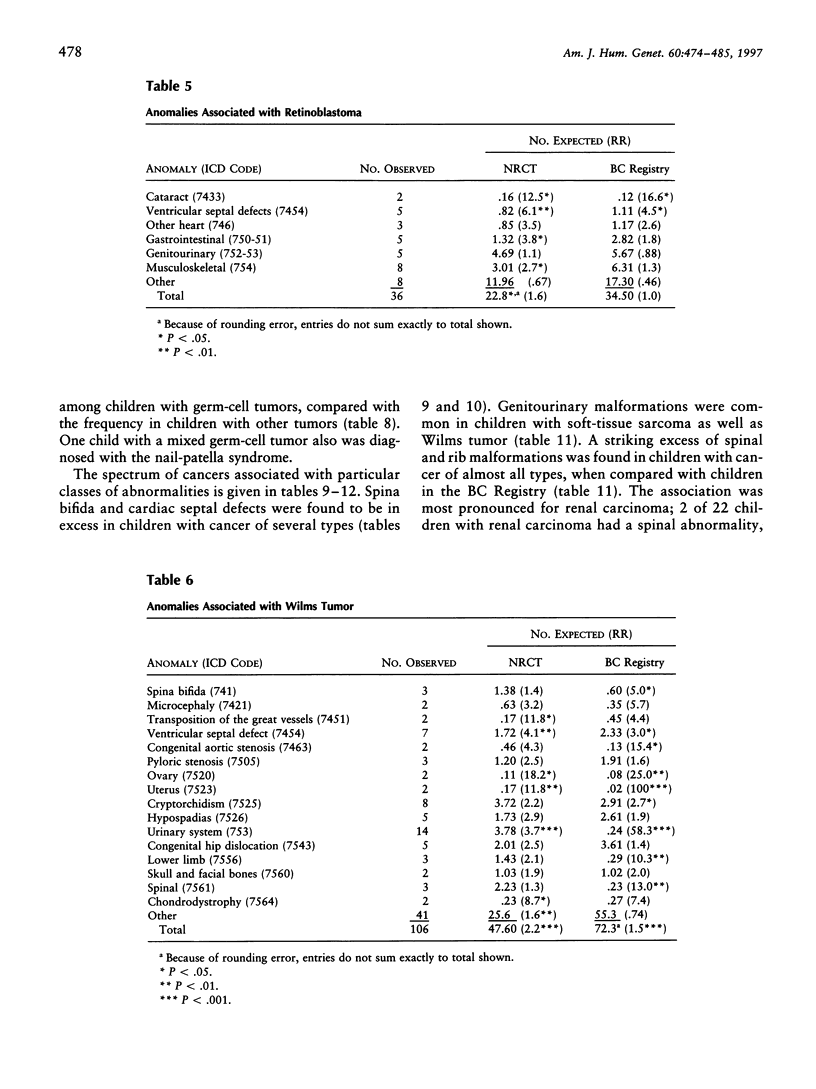
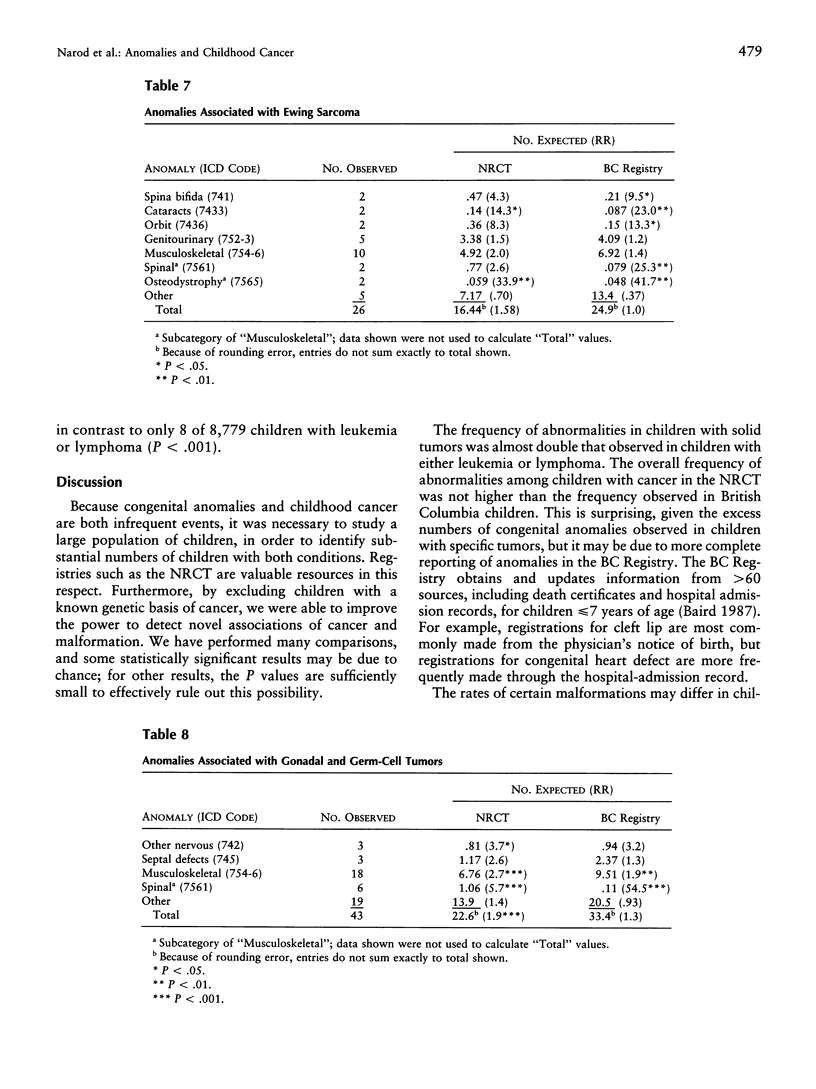
The presence of cancer and a congenital anomaly in the same child may be explained in certain cases by an underlying genetic abnormality. The study of these associations may lead to the identification of genes that are important in both processes. We have examined the records of 20,304 children with cancer in Britain, who were entered into the National Registry of Childhood Tumors (NRCT) during 1971-86, for the presence of congenital anomalies. The frequency of anomalies was much higher among children with solid tumors (4.4%) than among those with leukemia or lymphoma (2.6%; P < .0001). The types of cancer with the highest rates of anomalies were Wilms tumor (8.1%), Ewing sarcoma (5.8%), hepatoblastoma (6.4%), and gonadal and germ-cell tumors (6.4%). Cases of spina bifida and abnormalities of the eye, ribs, and spine were more common in children with cancer than among population-based controls. Future studies may be directed toward identifying the developmental pathways and the relevant genes that are involved in the overlap between pediatric cancer and malformation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwell J. D., Levick P. Congenital hypertrophic pyloric stenosis and associated anomalies in the genitourinary tract. J Pediatr Surg. 1981 Dec;16(6):1029–1035. doi: 10.1016/s0022-3468(81)80870-6. [DOI] [PubMed] [Google Scholar]
- Baird P. A. Measuring birth defects and handicapping disorders in the population: the British Columbia Health Surveillance Registry. CMAJ. 1987 Jan 15;136(2):109–111. [PMC free article] [PubMed] [Google Scholar]
- Baptiste M., Nasca P., Metzger B., Field N., MacCubbin P., Greenwald P., Armbrustmacher V., Waldman J., Carlton K. Neurofibromatosis and other disorders among children with CNS tumors and their families. Neurology. 1989 Apr;39(4):487–492. doi: 10.1212/wnl.39.4.487. [DOI] [PubMed] [Google Scholar]
- Barr F. G., Galili N., Holick J., Biegel J. A., Rovera G., Emanuel B. S. Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993 Feb;3(2):113–117. doi: 10.1038/ng0293-113. [DOI] [PubMed] [Google Scholar]
- Berry C. L., Keeling J., Hilton C. Coincidence of congenital malformation and embryonic tumours of childhood. Arch Dis Child. 1970 Apr;45(240):229–231. doi: 10.1136/adc.45.240.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch J. M., Marsden H. B. A classification scheme for childhood cancer. Int J Cancer. 1987 Nov 15;40(5):620–624. doi: 10.1002/ijc.2910400508. [DOI] [PubMed] [Google Scholar]
- Birch J. M., Marsden H. B., Swindell R. Pre-natal factors in the origin of germ cell tumours of childhood. Carcinogenesis. 1982;3(1):75–80. doi: 10.1093/carcin/3.1.75. [DOI] [PubMed] [Google Scholar]
- Blot W. J., Stiller C. A., Wilson L. M. Oral clefts and childhood cancer. Lancet. 1980 Mar 29;1(8170):722–722. [PubMed] [Google Scholar]
- Bonaïti-Pellié C., Chompret A., Tournade M. F., Hochez J., Moutou C., Zucker J. M., Steschenko D., Brunat-Mentigny M., Roché H., Tron P. Genetics and epidemiology of Wilms' tumor: the French Wilms' tumor study. Med Pediatr Oncol. 1992;20(4):284–291. doi: 10.1002/mpo.2950200404. [DOI] [PubMed] [Google Scholar]
- Bunin G. R., Kuijten R. R., Buckley J. D., Rorke L. B., Meadows A. T. Relation between maternal diet and subsequent primitive neuroectodermal brain tumors in young children. N Engl J Med. 1993 Aug 19;329(8):536–541. doi: 10.1056/NEJM199308193290804. [DOI] [PubMed] [Google Scholar]
- Chao L. Y., Huff V., Tomlinson G., Riccardi V. M., Strong L. C., Saunders G. F. Genetic mosaicism in normal tissues of Wilms' tumour patients. Nat Genet. 1993 Feb;3(2):127–131. doi: 10.1038/ng0293-127. [DOI] [PubMed] [Google Scholar]
- Evans G., Burnell L., Campbell R., Gattamaneni H. R., Birch J. Congenital anomalies and genetic syndromes in 173 cases of medulloblastoma. Med Pediatr Oncol. 1993;21(6):433–434. doi: 10.1002/mpo.2950210608. [DOI] [PubMed] [Google Scholar]
- Fraumeni J. F., Jr, Li F. P., Dalager N. Teratomas in children: epidemiologic features. J Natl Cancer Inst. 1973 Nov;51(5):1425–1430. doi: 10.1093/jnci/51.5.1425. [DOI] [PubMed] [Google Scholar]
- Herbst A. L., Ulfelder H., Poskanzer D. C. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971 Apr 15;284(15):878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- Klenerman L., Ockenden B. G., Townsend A. C. Osteosarcoma occurring in osteogenesis imperfecta. Report of two cases. J Bone Joint Surg Br. 1967 May;49(2):314–323. [PubMed] [Google Scholar]
- Lynch H. T., Green G. S. Wilms' tumor and congenital heart disease: report of a case and family. Am J Dis Child. 1968 Jun;115(6):723–727. doi: 10.1001/archpedi.1968.02100010725016. [DOI] [PubMed] [Google Scholar]
- MILLER R. W., FRAUMENI J. F., Jr, MANNING M. D. ASSOCIATION OF WILMS'S TUMOR WITH ANIRIDIA, HEMIHYPERTROPHY AND OTHER CONGENITAL MALFORMATIONS. N Engl J Med. 1964 Apr 30;270:922–927. doi: 10.1056/NEJM196404302701802. [DOI] [PubMed] [Google Scholar]
- Mann J. R., Dodd H. E., Draper G. J., Waterhouse J. A., Birch J. M., Cartwright R. A., Hartley A. L., McKinney P. A., Stiller C. A. Congenital abnormalities in children with cancer and their relatives: results from a case-control study (IRESCC). Br J Cancer. 1993 Aug;68(2):357–363. doi: 10.1038/bjc.1993.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J. R., Kasthuri N., Raafat F., Pincott J. R., Parkes S. E., Muir K. R., Ingram L. C., Cameron A. H. Malignant hepatic tumours in children: incidence, clinical features and aetiology. Paediatr Perinat Epidemiol. 1990 Jul;4(3):276–289. doi: 10.1111/j.1365-3016.1990.tb00651.x. [DOI] [PubMed] [Google Scholar]
- McKeen E. A., Hanson M. R., Mulvihill J. J., Glaubiger D. L. Birth defects with Ewing's sarcoma. N Engl J Med. 1983 Dec 15;309(24):1522–1522. doi: 10.1056/NEJM198312153092417. [DOI] [PubMed] [Google Scholar]
- Mili F., Khoury M. J., Flanders W. D., Greenberg R. S. Risk of childhood cancer for infants with birth defects. I. A record-linkage study, Atlanta, Georgia, 1968-1988. Am J Epidemiol. 1993 Mar 15;137(6):629–638. doi: 10.1093/oxfordjournals.aje.a116720. [DOI] [PubMed] [Google Scholar]
- Miller R. W., Rubinstein J. H. Tumors in Rubinstein-Taybi syndrome. Am J Med Genet. 1995 Mar 13;56(1):112–115. doi: 10.1002/ajmg.1320560125. [DOI] [PubMed] [Google Scholar]
- Mortlock D. P., Post L. C., Innis J. W. The molecular basis of hypodactyly (Hd): a deletion in Hoxa 13 leads to arrest of digital arch formation. Nat Genet. 1996 Jul;13(3):284–289. doi: 10.1038/ng0796-284. [DOI] [PubMed] [Google Scholar]
- Motegi T., Kaga M., Yanagawa Y., Kadowaki H., Watanabe K., Inoue A., Komatsu M., Minoda K. A recognizable pattern of the midface of retinoblastoma patients with interstitial deletion of 13q. Hum Genet. 1983;64(2):160–162. doi: 10.1007/BF00327116. [DOI] [PubMed] [Google Scholar]
- Mulinare J., Cordero J. F., Erickson J. D., Berry R. J. Periconceptional use of multivitamins and the occurrence of neural tube defects. JAMA. 1988 Dec 2;260(21):3141–3145. [PubMed] [Google Scholar]
- Mulvihill J. J., Gralnick H. R., Whang-Peng J., Leventhal B. G. Multiple childhood osteosarcomas in an American Indian family with erythroid macrocytosis and skeletal anomalies. Cancer. 1977 Dec;40(6):3115–3122. doi: 10.1002/1097-0142(197712)40:6<3115::aid-cncr2820400655>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Méhes K., Signer E., Plüss H. J., Müller H. J., Stalder G. Increased prevalence of minor anomalies in childhood malignancy. Eur J Pediatr. 1985 Sep;144(3):243–254. doi: 10.1007/BF00451951. [DOI] [PubMed] [Google Scholar]
- Narod S. A., Stiller C., Lenoir G. M. An estimate of the heritable fraction of childhood cancer. Br J Cancer. 1991 Jun;63(6):993–999. doi: 10.1038/bjc.1991.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Bruening W., Kashtan C. E., Mauer S. M., Manivel J. C., Striegel J. E., Houghton D. C., Junien C., Habib R., Fouser L. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell. 1991 Oct 18;67(2):437–447. doi: 10.1016/0092-8674(91)90194-4. [DOI] [PubMed] [Google Scholar]
- Petrij F., Giles R. H., Dauwerse H. G., Saris J. J., Hennekam R. C., Masuno M., Tommerup N., van Ommen G. J., Goodman R. H., Peters D. J. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature. 1995 Jul 27;376(6538):348–351. doi: 10.1038/376348a0. [DOI] [PubMed] [Google Scholar]
- Riccardi V. M., Sujansky E., Smith A. C., Francke U. Chromosomal imbalance in the Aniridia-Wilms' tumor association: 11p interstitial deletion. Pediatrics. 1978 Apr;61(4):604–610. [PubMed] [Google Scholar]
- Robison L. L., Swanson T., Day D. L., Ramsay N. K., L'Heureux P., Nesbit ME JrU Renal anomalies in childhood acute lymphoblastic leukemia. N Engl J Med. 1982 Oct 21;307(17):1086–1087. doi: 10.1056/NEJM198210213071722. [DOI] [PubMed] [Google Scholar]
- Ruymann F. B., Maddux H. R., Ragab A., Soule E. H., Palmer N., Beltangady M., Gehan E. A., Newton W. A., Jr Congenital anomalies associated with rhabdomyosarcoma: an autopsy study of 115 cases. A report from the Intergroup Rhabdomyosarcoma Study Committee (representing the Children's Cancer Study Group, the Pediatric Oncology Group, the United Kingdom Children's Cancer Study Group, and the Pediatric Intergroup Statistical Center). Med Pediatr Oncol. 1988;16(1):33–39. doi: 10.1002/mpo.2950160109. [DOI] [PubMed] [Google Scholar]
- Schumacher R., Mai A., Gutjahr P. Association of rib anomalies and malignancy in childhood. Eur J Pediatr. 1992 Jun;151(6):432–434. doi: 10.1007/BF01959357. [DOI] [PubMed] [Google Scholar]
- Stiller C. A., Allen M. B., Eatock E. M. Childhood cancer in Britain: the National Registry of Childhood Tumours and incidence rates 1978-1987. Eur J Cancer. 1995 Nov;31A(12):2028–2034. doi: 10.1016/0959-8049(95)00428-9. [DOI] [PubMed] [Google Scholar]
- Stiller C. A., Lennox E. L., Wilson L. M. Incidence of cardiac septal defects in children with Wilms' tumour and other malignant diseases. Carcinogenesis. 1987 Jan;8(1):129–132. doi: 10.1093/carcin/8.1.129. [DOI] [PubMed] [Google Scholar]
- Sy W. M., Edmonson J. H. The development defects associated with neuroblastoma--etiologic implications. Cancer. 1968 Jul;22(1):234–238. doi: 10.1002/1097-0142(196807)22:1<234::aid-cncr2820220127>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Tassabehji M., Read A. P., Newton V. E., Harris R., Balling R., Gruss P., Strachan T. Waardenburg's syndrome patients have mutations in the human homologue of the Pax-3 paired box gene. Nature. 1992 Feb 13;355(6361):635–636. doi: 10.1038/355635a0. [DOI] [PubMed] [Google Scholar]
- Weksberg R., Teshima I., Williams B. R., Greenberg C. R., Pueschel S. M., Chernos J. E., Fowlow S. B., Hoyme E., Anderson I. J., Whiteman D. A. Molecular characterization of cytogenetic alterations associated with the Beckwith-Wiedemann syndrome (BWS) phenotype refines the localization and suggests the gene for BWS is imprinted. Hum Mol Genet. 1993 May;2(5):549–556. doi: 10.1093/hmg/2.5.549. [DOI] [PubMed] [Google Scholar]
- Windham G. C., Bjerkedal T., Langmark F. A population-based study of cancer incidence in twins and in children with congenital malformations or low birth weight, Norway, 1967-1980. Am J Epidemiol. 1985 Jan;121(1):49–56. doi: 10.1093/oxfordjournals.aje.a113982. [DOI] [PubMed] [Google Scholar]


