Abstract
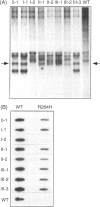
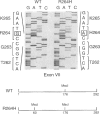
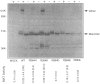
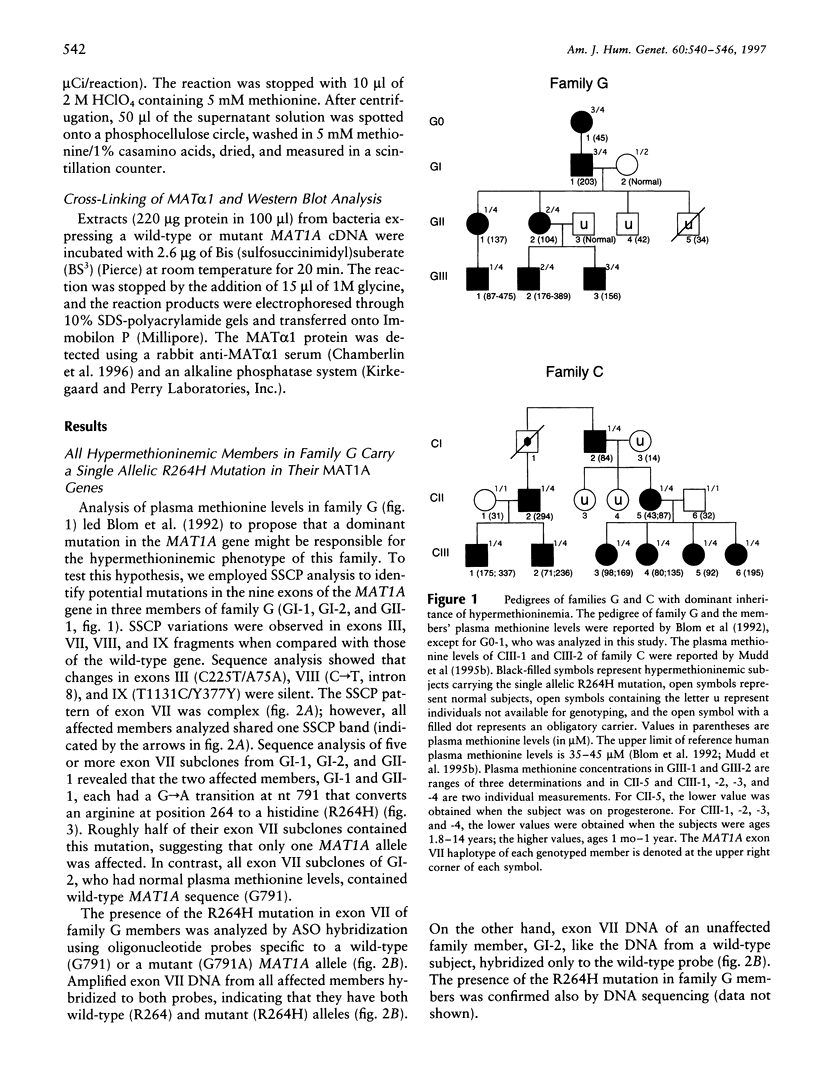
Methionine adenosyltransferase (MAT) I/III deficiency, characterized by isolated persistent hypermethioninemia, is caused by mutations in the MAT1A gene encoding MAT(alpha)1, the subunit of major hepatic enzymes MAT I ([alpha1]4) and III([alpha1]2). We have characterized 10 MAT1A mutations in MAT I/III-deficient individuals and shown that the associated hypermethioninemic phenotype was inherited as an autosomal recessive trait. However, dominant inheritance of hypermethioninemia, also hypothesized to be caused by MAT I/III deficiency, has been reported in two families. Here we show that the only mutation uncovered in one of these families, G, is a G-->A transition at nt 791 in exon VII of one MAT1A allele that converts an arginine at position 264 to a histidine (R264H). This single allelic R264H mutation was subsequently identified in two hypermethioninemic individuals in an additional family, C. Family C members were also found to inherit hypermethioninemia in a dominant fashion, and the available affected members analyzed carried the single allelic R264H mutation. Substitution of R-264 with histidine (R264H, the naturally occurring mutant), leucine (R264L), aspartic acid (R264D), or glutamic acid (R264E) greatly reduced MAT activity and severely impaired the ability of the MAT(alpha)1 subunits to form homodimers essential for optimal catalytic activity. On the other hand, when lysine was substituted for R-264 (R264K), the mutant alpha1 subunit was able to form dimers that retain significant MAT activity, suggesting that amino acid 264 is involved in intersubunit salt-bridge formation. Cotransfection studies show that R264/R264H MAT(alpha)1 heterodimers are enzymatically inactive, thus providing an explanation for the R264H-mediated dominant inheritance of hypermethioninemia.
Full text
PDF

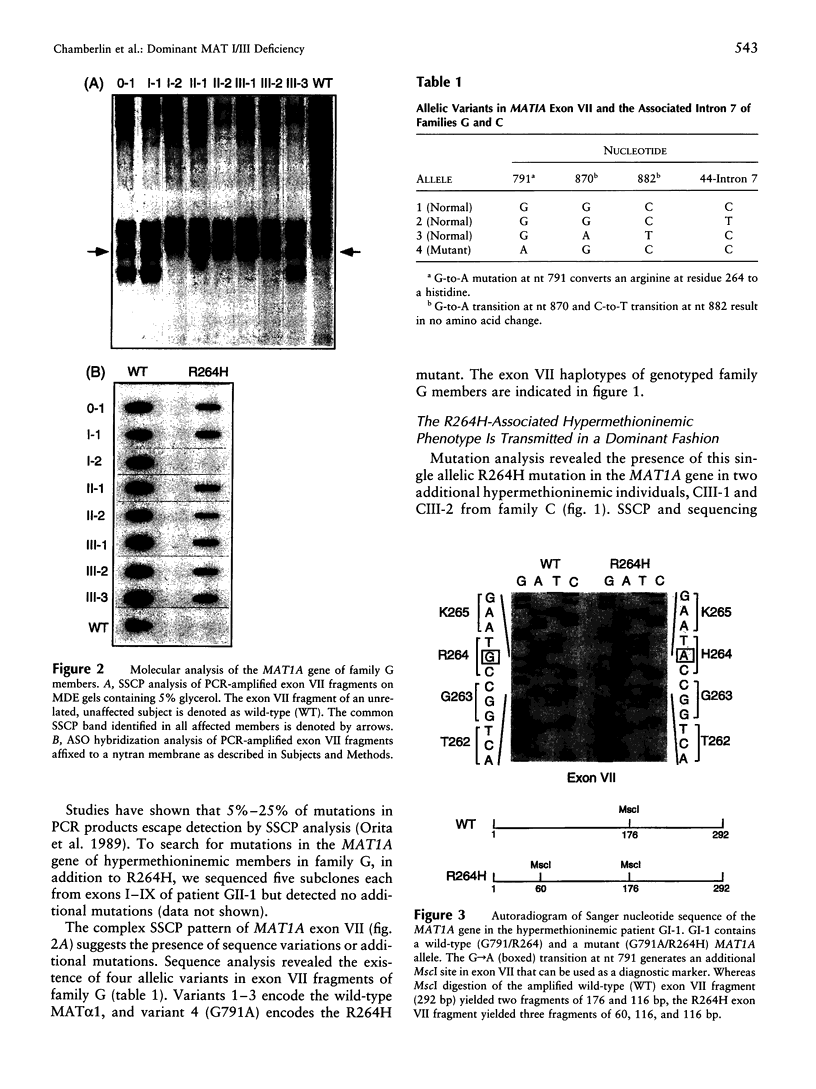
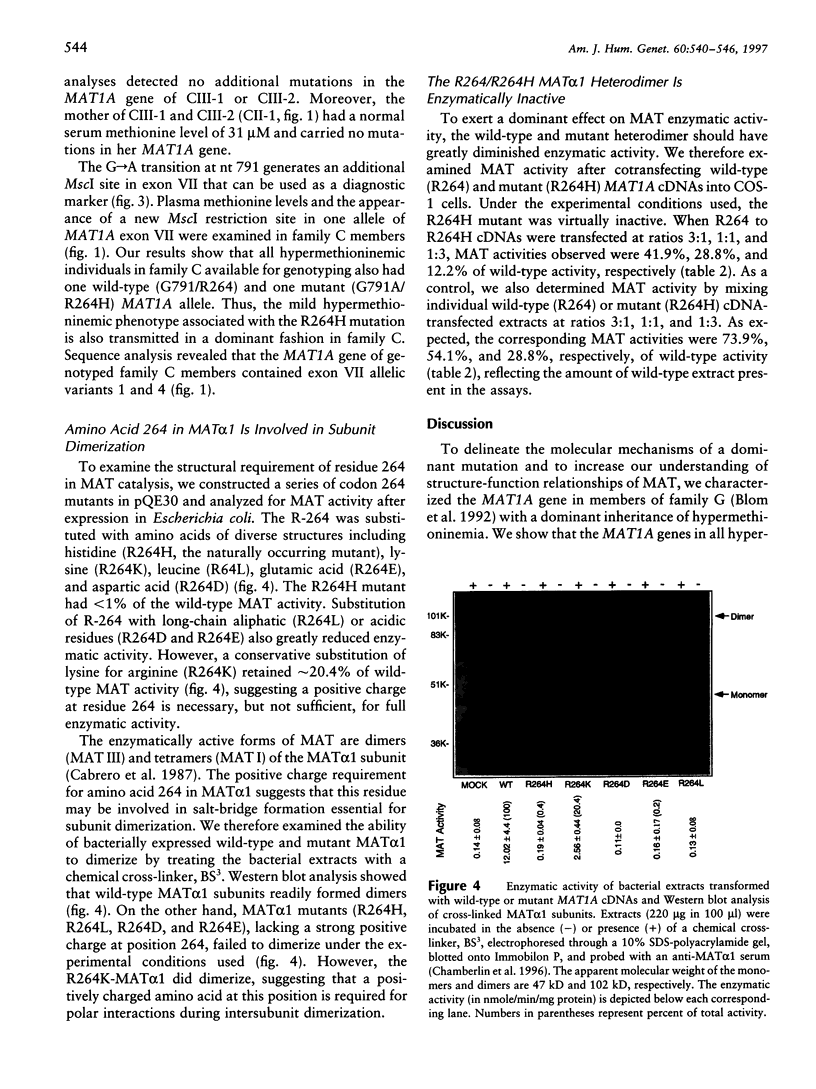


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blom H. J., Davidson A. J., Finkelstein J. D., Luder A. S., Bernardini I., Martin J. J., Tangerman A., Trijbels J. M., Mudd S. H., Goodman S. I. Persistent hypermethioninaemia with dominant inheritance. J Inherit Metab Dis. 1992;15(2):188–197. doi: 10.1007/BF01799629. [DOI] [PubMed] [Google Scholar]
- CATONI G. L. S-Adenosylmethionine; a new intermediate formed enzymatically from L-methionine and adenosinetriphosphate. J Biol Chem. 1953 Sep;204(1):403–416. [PubMed] [Google Scholar]
- Cabrero C., Puerta J., Alemany S. Purification and comparison of two forms of S-adenosyl-L-methionine synthetase from rat liver. Eur J Biochem. 1987 Dec 30;170(1-2):299–304. doi: 10.1111/j.1432-1033.1987.tb13699.x. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. E., Ubagai T., Mudd S. H., Wilson W. G., Leonard J. V., Chou J. Y. Demyelination of the brain is associated with methionine adenosyltransferase I/III deficiency. J Clin Invest. 1996 Aug 15;98(4):1021–1027. doi: 10.1172/JCI118862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Rosa J., Ostrowski J., Hryniewicz M. M., Kredich N. M., Kotb M., LeGros H. L., Jr, Valentine M., Geller A. M. Chromosomal localization and catalytic properties of the recombinant alpha subunit of human lymphocyte methionine adenosyltransferase. J Biol Chem. 1995 Sep 15;270(37):21860–21868. doi: 10.1074/jbc.270.37.21860. [DOI] [PubMed] [Google Scholar]
- Finkelstein J. D., Kyle W. E., Martin J. J. Abnormal methionine adenosyltransferase in hypermethioninemia. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1491–1497. doi: 10.1016/0006-291x(75)90527-6. [DOI] [PubMed] [Google Scholar]
- Gahl W. A., Bernardini I., Finkelstein J. D., Tangerman A., Martin J. J., Blom H. J., Mullen K. D., Mudd S. H. Transsulfuration in an adult with hepatic methionine adenosyltransferase deficiency. J Clin Invest. 1988 Feb;81(2):390–397. doi: 10.1172/JCI113331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaull G. E., Tallan H. H., Lonsdale D., Przyrembel H., Schaffner F., von Bassewitz D. B. Hypermethioninemia associated with methionine adenosyltransferase deficiency: clinical, morphologic, and biochemical observations on four patients. J Pediatr. 1981 May;98(5):734–741. doi: 10.1016/s0022-3476(81)80833-5. [DOI] [PubMed] [Google Scholar]
- Horikawa S., Sasuga J., Shimizu K., Ozasa H., Tsukada K. Molecular cloning and nucleotide sequence of cDNA encoding the rat kidney S-adenosylmethionine synthetase. J Biol Chem. 1990 Aug 15;265(23):13683–13686. [PubMed] [Google Scholar]
- Horikawa S., Tsukada K. Molecular cloning and developmental expression of a human kidney S-adenosylmethionine synthetase. FEBS Lett. 1992 Nov 2;312(1):37–41. doi: 10.1016/0014-5793(92)81405-b. [DOI] [PubMed] [Google Scholar]
- Kotb M., Geller A. M. Methionine adenosyltransferase: structure and function. Pharmacol Ther. 1993 Aug;59(2):125–143. doi: 10.1016/0163-7258(93)90042-c. [DOI] [PubMed] [Google Scholar]
- Kotb M., Kredich N. M. S-Adenosylmethionine synthetase from human lymphocytes. Purification and characterization. J Biol Chem. 1985 Apr 10;260(7):3923–3930. [PubMed] [Google Scholar]
- Mitsui K., Teraoka H., Tsukada K. Complete purification and immunochemical analysis of S-adenosylmethionine synthetase from bovine brain. J Biol Chem. 1988 Aug 15;263(23):11211–11216. [PubMed] [Google Scholar]
- Mudd S. H., Levy H. L., Tangerman A., Boujet C., Buist N., Davidson-Mundt A., Hudgins L., Oyanagi K., Nagao M., Wilson W. G. Isolated persistent hypermethioninemia. Am J Hum Genet. 1995 Oct;57(4):882–892. [PMC free article] [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata S. F., Shelly L. L., Ruppert S., Schutz G., Chou J. Y. Cloning and expression of murine S-adenosylmethionine synthetase. J Biol Chem. 1993 Jul 5;268(19):13978–13986. [PubMed] [Google Scholar]
- Surtees R., Leonard J., Austin S. Association of demyelination with deficiency of cerebrospinal-fluid S-adenosylmethionine in inborn errors of methyl-transfer pathway. Lancet. 1991 Dec 21;338(8782-8783):1550–1554. doi: 10.1016/0140-6736(91)92373-a. [DOI] [PubMed] [Google Scholar]
- Takusagawa F., Kamitori S., Markham G. D. Structure and function of S-adenosylmethionine synthetase: crystal structures of S-adenosylmethionine synthetase with ADP, BrADP, and PPi at 28 angstroms resolution. Biochemistry. 1996 Feb 27;35(8):2586–2596. doi: 10.1021/bi952604z. [DOI] [PubMed] [Google Scholar]
- Takusagawa F., Kamitori S., Misaki S., Markham G. D. Crystal structure of S-adenosylmethionine synthetase. J Biol Chem. 1996 Jan 5;271(1):136–147. [PubMed] [Google Scholar]
- Ubagai T., Lei K. J., Huang S., Mudd S. H., Levy H. L., Chou J. Y. Molecular mechanisms of an inborn error of methionine pathway. Methionine adenosyltransferase deficiency. J Clin Invest. 1995 Oct;96(4):1943–1947. doi: 10.1172/JCI118240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. A., Vasser M., Powers D. B. Cassette mutagenesis: an efficient method for generation of multiple mutations at defined sites. Gene. 1985;34(2-3):315–323. doi: 10.1016/0378-1119(85)90140-4. [DOI] [PubMed] [Google Scholar]